Abstract
Nalbuphine (Nubain®) is a mixed action mu-kappa agonist used clinically for the management of pain. Nalbuphine and other mu-kappa agonists decreased cocaine self-administration in preclinical models. Cocaine stimulates the hypothalamic-pituitary-adrenal (HPA) axis, but the effects of nalbuphine on the HPA axis are unknown. Analgesic doses (5 and 10 mg/70 kg) of IV nalbuphine were administered to healthy male cocaine abusers, and plasma levels of PRL, ACTH and cortisol were measured before and at 10, 17, 19, 23, 27, 31, 35, 40, 45, 60, 75, 105, 135 min after nalbuphine administration. Subjective effects were measured on a Visual Analog Scale (VAS). Prolactin (PRL) increased significantly within 17 min (P=.04) and reached peak levels of 22.1 ± 7.1 ng/ml and 54.1 ± 11.3 at 60 min after low and high dose nalbuphine administration, respectively. VAS reports of “Sick,” “Bad” and “Dizzy” were significantly higher after 10 mg/70 kg than after 5 mg/70 kg nalbuphine (P=.05−.0001), and were significantly correlated with increases in PRL (P=.05−.0003). However, sedation and emesis were observed only after a 10 mg/70 kg dose of nalbuphine. Interestingly, ACTH and cortisol levels did not change significantly after administration of either dose of nalbuphine. Taken together, these data suggest that nalbuphine had both mu- and kappa-like effects on PRL (PRL increase) but did not increase ACTH and cortisol.
Introduction
Nalbuphine hydrochloride (Nubain®) is a synthetic opioid analgesic that is chemically related to the potent opioid analgesic, oxymorphone, and the widely used opioid antagonist, naloxone. The analgesic potency of nalbuphine is essentially equivalent to that of morphine on a weight basis (Errick and Heel, 1983; Miller, 1980; Zacny et al., 1997). Nalbuphine is used primarily for the management of pre- and postoperative pain, but is also used as an analgesic during labor and delivery (Cohen et al., 1992), after myocardial infarction (Jamidar et al., 1987), and as a parenteral analgesic by paramedics in the prehospital setting (Woollard et al., 2002). Administration of nalbuphine causes some respiratory depression similar to that induced by morphine (Lake et al., 1984). However, unlike morphine, administration of higher doses of nalbuphine does not appreciably increase the respiratory depression (Romagnoli and Keats, 1980). This ceiling effect makes the drug a valuable adjunct to inhaled anesthetics during various surgical procedures, especially those requiring stabilization of hemodynamic states, such as cardiac and valvular surgeries.
Nalbuphine has affinity for both mu and kappa opioid receptors in the CNS and is traditionally classified as a mixed-action mu antagonist/kappa agonist (Jaffe and Martin, 1990; PDR, 2003; Preston and Jasinski, 1991). Studies in mice suggest that nalbuphine produces analgesia through an interaction between the supraspinal kappa 3 receptor and the spinal kappa 1 receptor (Pick et al., 1992). Direct activation of kappa receptors by nalbuphine has been demonstrated in vitro in cells that express cloned human kappa opioid receptors (Zhu et al., 1997). Furthermore, in vitro data demonstrated that nalbuphine is also an agonist at mu-receptors with a variable efficacy similar to that of morphine (Emmerson et al., 1996; Gharagozlou et al., 2003).
Nalbuphine was approved by the FDA as a nonscheduled opioid analgesic in the United States in 1979. Although this opioid is generally regarded as having low abuse liability, subjective evaluations by human subjects (Bigelow et al., 1984), as well as studies of drug self-administration in nonhuman primates (Lukas et al., 1986), suggest that the abuse potential of nalbuphine is comparable to that of other opioids. Indeed, nalbuphine dependence has been described in three users of anabolic steroids in Britain (McBride et al., 1996), and studies in the United States suggest that nalbuphine abuse or dependence among male and female athletes may reflect a more widespread phenomenon than previously believed (Wines et al., 1999). Users report taking nalbuphine to treat pain from injuries, to continue training despite musculoskeletal pain, and to keep calm before and during athletic events. The major symptoms of nalbuphine withdrawal reported were abdominal cramps, sweating, tremors, and irritability (McBride et al., 1996; Wines et al., 1999).
Opioid use is associated with perturbations in the hypothalamic-pituitary-adrenal (HPA) axis and/or the hypothalamic-pituitary-gonadal (HPG) axis. Mu and kappa opioids perturb the neuroendocrine system and influence the release of the anterior pituitary and adrenal hormones (Grossman, 1983). Clinical studies in healthy volunteers have shown that kappa opioid receptor-selective agonists increase serum levels of PRL (Ur et al., 1997), but their effects on cortisol are inconsistent and both increases in cortisol levels and decreases in cortisol and ACTH levels have been reported (Ur et al., 1997). Pfeiffer and co-workers (Pfeiffer et al., 1986) concluded that a kappa opioid agonist inhibited ACTH and cortisol secretion in a naloxone-reversible manner. However, in 5 other subjects, there was an unexpected large increase in ACTH and cortisol secretion following kappa agonist administration (Pfeiffer et al., 1986). Furthermore, Ur et al. 1997 reported a dose-dependent stimulation of cortisol release by kappa-selective agonist spiradoline in healthy human volunteers. Kappa opioid antagonists, such as nor-BNI, tend to decrease PRL in rats (Baumann and Rabii, 1991; Manzanares et al., 1993) and increase ACTH and cortisol in rhesus monkeys (Williams et al., 2003). The mu antagonists naloxone and naltrexone, and the mixed mu/kappa antagonist nalmefene, increase ACTH and cortisol in human subjects (Hernandez-Avila et al., 2002; Mendelson et al., 1986 Mendelson et al., 1991; Schluger et al., 1998; Teoh et al., 1988; Wand et al., 1999a, b). Mu opioid agonists also stimulate PRL release under some conditions (Bowen et al., 2002; Hoehe et al., 1988; Saarialho-Kere et al., 1989), but inhibit ACTH and cortisol (Auernhammer et al., 1992, 1994). In contrast, selective mu-antagonists such as beta-FNA decrease PRL in rats (Baumann and Rabii, 1991; Koenig et al., 1984) and block morphine-induced ACTH release in rats (Pfeiffer et al., 1985). In rhesus monkeys, the mu antagonist quadazocine did not alter PRL levels significantly but antagonized the heroin-induced increase in PRL (Bowen et al., 2002).
PRL regulation is very complex and involves both neuroendocrine and paracrine mechanisms (Ben-Jonathan, 1985). PRL is under inhibitory control by dopamine and dopamine antagonists stimulate PRL release (Ben-Jonathan and Hnasko, 2001). This study was part of a series designed to evaluate the effects of nalbuphine on the abuse-related effects of cocaine (Mello et al., 2005). Preclinical studies suggest that mixed action mu/kappa opioids selectively reduce cocaine self-administration in non-human primates (Bowen et al., 2003; Mello et al., 1993; Mello and Negus, 2000). However, the neuroendocrine and subjective effects associated with acute administration of the mixed-action kappa agonist/mu antagonist, nalbuphine, in humans have not been studied extensively. The purpose of the present study was to investigate the acute effects of nalbuphine on anterior pituitary hormones (PRL, ACTH) and the adrenal hormone cortisol, and to determine the covariance between hormone changes and reports of subjective effects in men.
Methods
Subjects
Fifteen male volunteers who fulfilled DSM-IV criteria for current cocaine abuse (305.6) provided written informed consent for participation in this study. All subjects were recruited via local newspaper advertisements and were paid for their participation. The study was approved by the Institutional Review Board of McLean Hospital. Volunteers with any lifetime DSM-IV Axis I disorder other than cocaine abuse and nicotine dependence (305.1) were excluded. All men selected had normal physical and laboratory screening profiles and were in good health. Over the course of the study, four subjects withdrew from participating for personal reasons and one subject moved away. As a consequence, data are presented for ten subjects. Subject confidentiality was protected by a Confidentiality Certificate from the National Institute on Drug Abuse (NIDA), and the study was conducted in compliance with Health Insurance Portability and Accountability Act (HIPAA) health privacy regulations. Table 1 summarizes the subjects’ characteristics. Subjects did not differ significantly with respect to age or BMI.
Table 1.
Subject characteristics Values are mean ± S.E.M.
| Age | Education | BMI | |
|---|---|---|---|
| 5mg/70kg(n=7) | 26.1±1.6 | 13.0±0.7 | 25.1±1.1 |
| 10mg/70kg(n=3) | 26.0±1.5 | 13.3±2.0 | 24.5±2.1 |
Rationale for Nalbuphine Selection
The analgesic potency of nalbuphine is essentially equivalent to that of morphine and the usual recommended adult analgesic dose is 10 mg/70 kg (PDR, 2005). In normal healthy volunteers, 10 mg/70 kg nalbuphine and morphine have been shown to produce similar subjective and physiological effects (Zacny et al., 1997). However, in cocaine abusers, 10 mg/70 kg nalbuphine produced emesis and sedation, so only three subjects were studied at this dose. A lower dose of 5 mg/70 kg nalbuphine was administered to seven subjects and produced no adverse reactions. Nalbuphine doses of 5 or 6 mg/70 kg have proven to be safe and to induce changes in positive subjective effects (e.g., High, Like Drug Effects, Feel Drug Effect) (Preston and Bigelow, 2000; Zacny et al., 1997). These positive subjective effects after 5mg/70kg nalbuphine administration were also reported by Mello et al., 2005, using the methodology described in this paper. Specifically, within 10–25 minutes after nalbuphine administration, Visual Analog Scale (VAS) ratings increased significantly for measures of High, Stimulated, Good Effect, Drug Effect, and Euphoria and remained significantly above baseline measures for 45–135 minutes (Mello et al., 2005).
Nalbuphine Preparation
The nalbuphine dose was prepared by diluting Nubain® (20 mg/ml, Endo Pharmaceuticals Inc., Chadds Ford, PA) with 0.9% sterile saline. The nalbuphine dose was based on body weight and is expressed as milligrams of the salt weight per 70-kg body weight.
Drug Abstinence Requirements
Subjects were tested for recent drug use before nalbuphine administration. On the morning of each study day, subjects provided a urine sample for analysis with the Triage® Drugs of Abuse (DOA) Panel (Biosite Diagnostics, San Diego, CA). The Triage® DOA Panel qualitatively detects the presence of the following drugs of abuse (or their metabolites) in urine at the designated cut-off concentrations recommended by the Substance Abuse and Mental Health Services Administration: phencyclidine 25 ng/mL, benzodiazepines 300 ng/mL, benzylecgonine (a metabolite of cocaine) 300 ng/mL, amphetamines 1000 ng/mL, tetrahydrocannabinol 50 ng/mL, opiates 300 ng/mL and barbiturates 300 ng/mL. No subject tested positive for any of these drugs or their metabolites. Subjects were given a breath alcohol test (Alco-Sensor IV, Intoximeters, Inc., St. Louis, MO) to ensure that they had not been drinking alcohol-containing beverages recently. They were asked to abstain from smoking and caffeinated beverages after midnight before the study. Subjects were also required not to eat food or drink any non-clear liquids for 4 h, and not to drink clear liquids for 2 h before the study session. Carbon monoxide (CO) levels were measured with a Vitalograph Breath CO Monitor from Vitalograph, Inc. (Lenexa, KS) to assess compliance with smoking abstinence requirements. Subjects with a CO level above 10 ppm were excluded.
Experimental Conditions
Studies were carried out on a clinical research ward used exclusively for investigations of substance abuse. Only one subject was studied on each experimental day. Subjects sat in a comfortable chair in front of a computer that was used to collect subjective responses during the test session. Subjective responses and physiological data were collected before nalbuphine administration and after nalbuphine administration. Each test session lasted for 150 min.
After subjects completed the baseline subjective-effects questionnaires, nalbuphine (5 or 10 mg/70 kg) was administered into the subject’s antecubital vein over 15 s. Saline was administered 12 min after the nalbuphine injection. Heart rate, pulse oximetry were monitored throughout the study session with a noninvasive patient monitor model (Scholar II/507E, Criticare Systems, Waukesha, WI). Systolic and diastolic blood pressures were measured periodically at baseline, and 10, 20, 25, 30, 35, 40, 45, 60, 75, 105 and 135 min after nalbuphine injection. Vital signs were monitored for at least 4 h after completion of the study. A physician certified in cardiopulmonary resuscitation was present during each study, and a cardiac defibrillator and appropriate emergency treatment medications were located in the study room.
Sample Collection Procedures
All samples for nalbuphine and hormone analysis were collected from an intravenous catheter placed in the antecubital vein of the arm opposite the arm used for nalbuphine injection. Baseline blood samples for analysis of serum drug levels were collected 30 min before nalbuphine injection. Samples for analysis of serum nalbuphine levels were collected at 10, 20, 25, 45, and 135 min after nalbuphine injection. Samples for serum/plasma analysis of PRL, ACTH, and cortisol, were obtained 30 min and 5 min before drug administration and at 10, 17, 19, 23, 27, 31, 35, 40, 45, 60, 75, 105, 135 min after nalbuphine injection. Subjective measures included a Visual Analog Scale (VAS), a Subject-Rated Adjective Checklist, and the short form of the Addiction Research Center Inventory (ARCI) (Martin et al., 1971), and a Subject-Rated Adjective Checklist. Items included on the VAS and the Adjective Checklist were based on previous clinical studies of opioid effects and cocaine effects (Foltin and Fischman, 1992; Preston et al., 1989; Walsh et al., 2001b; Zacny et al., 1997). Subjective effects ratings were collected at baseline and periodically after nalbuphine administration. Specifically, VAS ratings were completed at 10, 20, 25, 30, 35, 40, 45, 60, 75, 105 and 135 min after nalbuphine injection. Subjects were asked to rate the following drug effects: “Sick”, “Dizzy”, “Bad” Effect. These items were selected to reflect opioid effects reported in previous studies (Foltin et al., 1995; Walsh et al., 1996). Each of the items was rated from 0 (not at all) to 100 (extremely) on a 100 mm line on the computer screen. The VAS ratings were usually completed within 1–2 min.
Assay procedures
PRL Assay
Serum PRL was determined in duplicate by the ImmuChem hPRL IRMA method, using kits (Cat. #: 07-274-102) purchased from ICN Biomedicals, Inc. (Costa Mesa, CA). The assay sensitivity was 0.1 ng/ml and the intra- and interassay C. V.’s were 1.3% and 6.0%, respectively.
ACTH Assay
Plasma ACTH was determined in duplicate by IRMA method using Alegro kits (Cat.#: 40-2195) purchased from Nichols Institute Diagnostics (San Juan Capistrano, CA). The assay sensitivity was 1.7 pg/ml and the intra- and interassay C.V.’s were 3.9% and 5.6%, respectively.
Cortisol Assay
Plasma cortisol was determined in duplicate by the GammaCoat RIA method, using kits (Cat. #: CA-1529) purchased from DiaSorin Corporation (Stillwater, MN). The assay sensitivity was 0.2 pg/dl and the intra- and interassay C. V.’s were 5.9% and 9.6%, respectively.
Serum Nalbuphine Analysis
Serum concentrations of nalbuphine were measured in duplicate using a solid phase ELISA purchased from Neogen Corporation (Lexington, KY), with nalbuphine hydrochloride dehydrate standard from Sigma-Aldrich (St. Louis, MO). The assay sensitivity was 0.06 ng/ml and the intra- and interassay C.V.s were 5.4% and 6.5%, respectively.
Data Analysis
Data were analyzed by using a repeated measures ANOVA. If significant main effects were detected, one-way ANOVAs were performed to identify the time points that differed significantly from baseline within each group. Comparisons between the effects of 5 mg nalbuphine/70 kg and 10 mg nalbuphine/70 kg were also analyzed with ANOVA for repeated measures.
Results
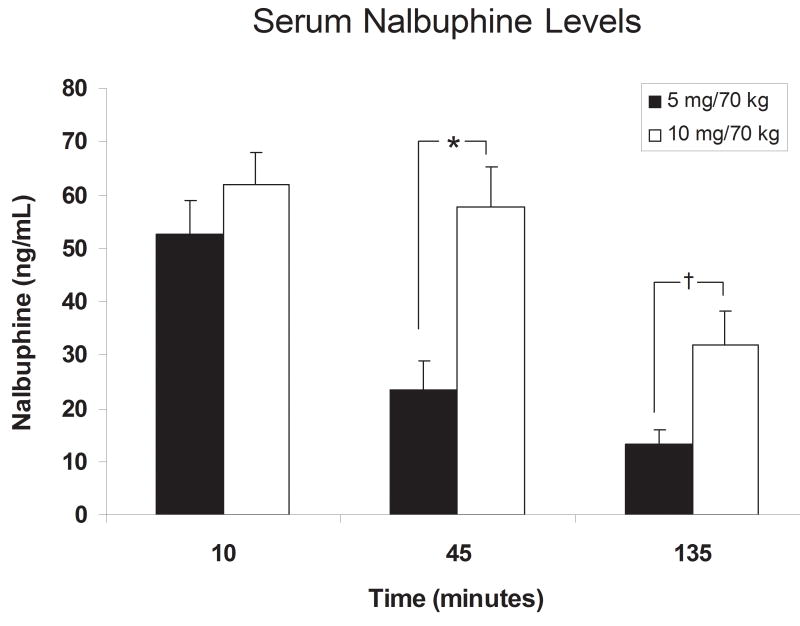
Effect of Nalbuphine Administration on Serum Nalbuphine Levels (Figure 1)
Fig. 1.
Serum nalbuphine levels after IV administration of 5 or 10 mg/kg nalbuphine. Significant differences are indicated by asterisks (*, P < 0.05) or daggers (†, P < 0.001). 5 mg nalbuphine pharmacokinetic data were from our earlier behavioral study (for reference see Mello et al., 2005).
Serum nalbuphine levels were measured 10, 45 and 135 minutes after nalbuphine administration (Fig. 1). Ten minutes after nalbuphine administration, serum nalbuphine levels averaged 52.5 ± 6.5 and 61.9 ± 6.2 ng/ml in the subjects who received 5 and 10 mg nalbuphine/70 kg, respectively, but did not differ between groups. At 45 and 135 minutes after nalbuphine administration, serum nalbuphine levels were significantly higher in subjects who received 10 mg/70 kg of nalbuphine.
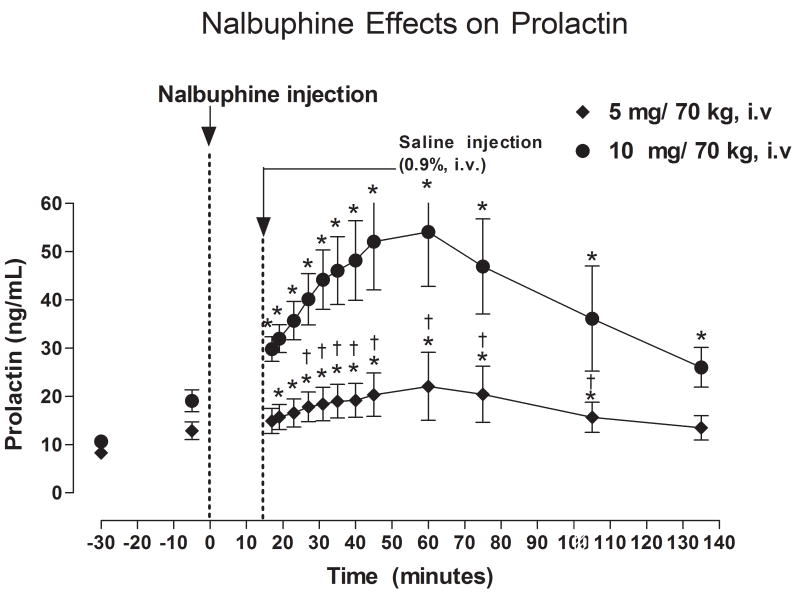
Effect of Nalbuphine on Serum PRL Levels (Figure 2)
Fig. 2.
Serum PRL levels after IV administration of 5 or 10 mg/kg nalbuphine. Significant changes from the pre-nalbuphine baseline are indicated by asterisks (*, P < 0.05). Daggers (†, P <0.05) indicate points at which PRL levels differed between subjects who received 5 or 10 mg nalbuphine.
Before nalbuphine administration, there were no significant differences in PRL levels between the two nalbuphine dose groups; baseline PRL levels averaged 8.31 ± 0.77 and 10.63 ± 1.22 ng/ml, respectively (Fig. 2). Administration of 5 mg nalbuphine/70 kg IV significantly increased serum PRL levels within 17 minutes after injection (P < 0.05). Serum PRL levels remained significantly higher than baseline for 105 minutes post-nalbuphine injection, but returned to baseline levels within 135 minutes. Peak PRL levels of 22.1 ng/ml (165% higher than baseline) were measured 60 minutes after nalbuphine administration. Administration of 10 mg nalbuphine/70 kg also increased serum PRL levels significantly within 17 minutes after injection (P < 0.05), and PRL levels remained higher than baseline at all subsequent time points. Peak PRL levels of 54.1 ng/ml (400% higher than baseline) were measured 60 minutes after administration of 10 mg nalbuphine/70 kg. PRL levels at 27, 31, 35, 40, 45, 60, and 75, and 105 minutes post-nalbuphine injection were significantly higher in subjects who received 10 mg nalbuphine/70 kg than those who received 5 mg nalbuphine/70 kg (P < 0.05−0.01).
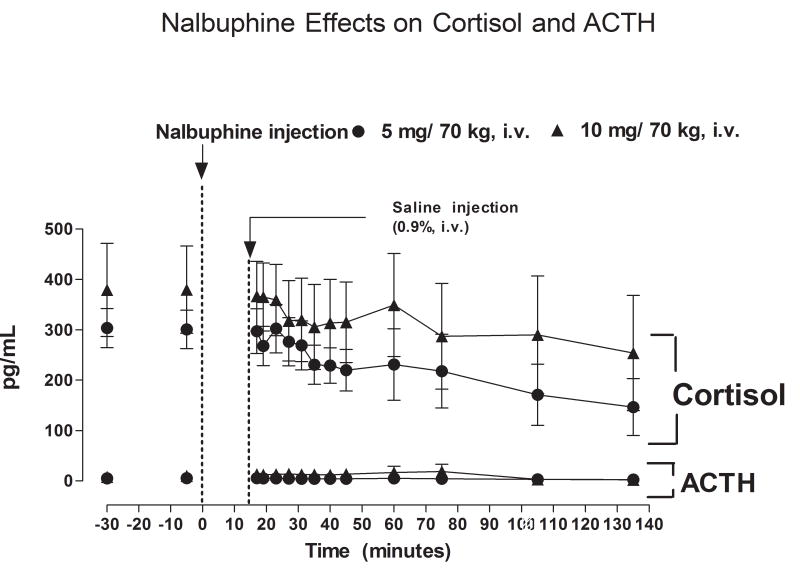
Effect of Nalbuphine on Plasma ACTH and Cortisol (Figure 3)
Fig. 3.
Plasma ACTH and cortisol levels after IV administration of 5 or 10 mg/kg nalbuphine.
Baseline levels ACTH and cortisol did not differ significantly between the two nalbuphine dose groups. Prior to nalbuphine injection, average baseline levels of ACTH were 4.82 ± 0.34 and 7.81 ± 0.84 pg/ml in subjects who received 5 or 10 mg nalbuphine/70 kg, respectively. Plasma ACTH levels did not change significantly over the course of the study period after administration of either dose of nalbuphine (Fig. 3). Average baseline plasma cortisol levels were 303.3 ± 38.7 and 379.3 ± 92.6 pg/ml in subjects who received 5 or 10 mg nalbuphine/70 kg, respectively. Plasma cortisol levels tended to decrease after nalbuphine injection, but the decline was not statistically significant (Fig. 3). Plasma cortisol levels did not differ between subjects who received 5 or 10 mg/70 kg nalbuphine.
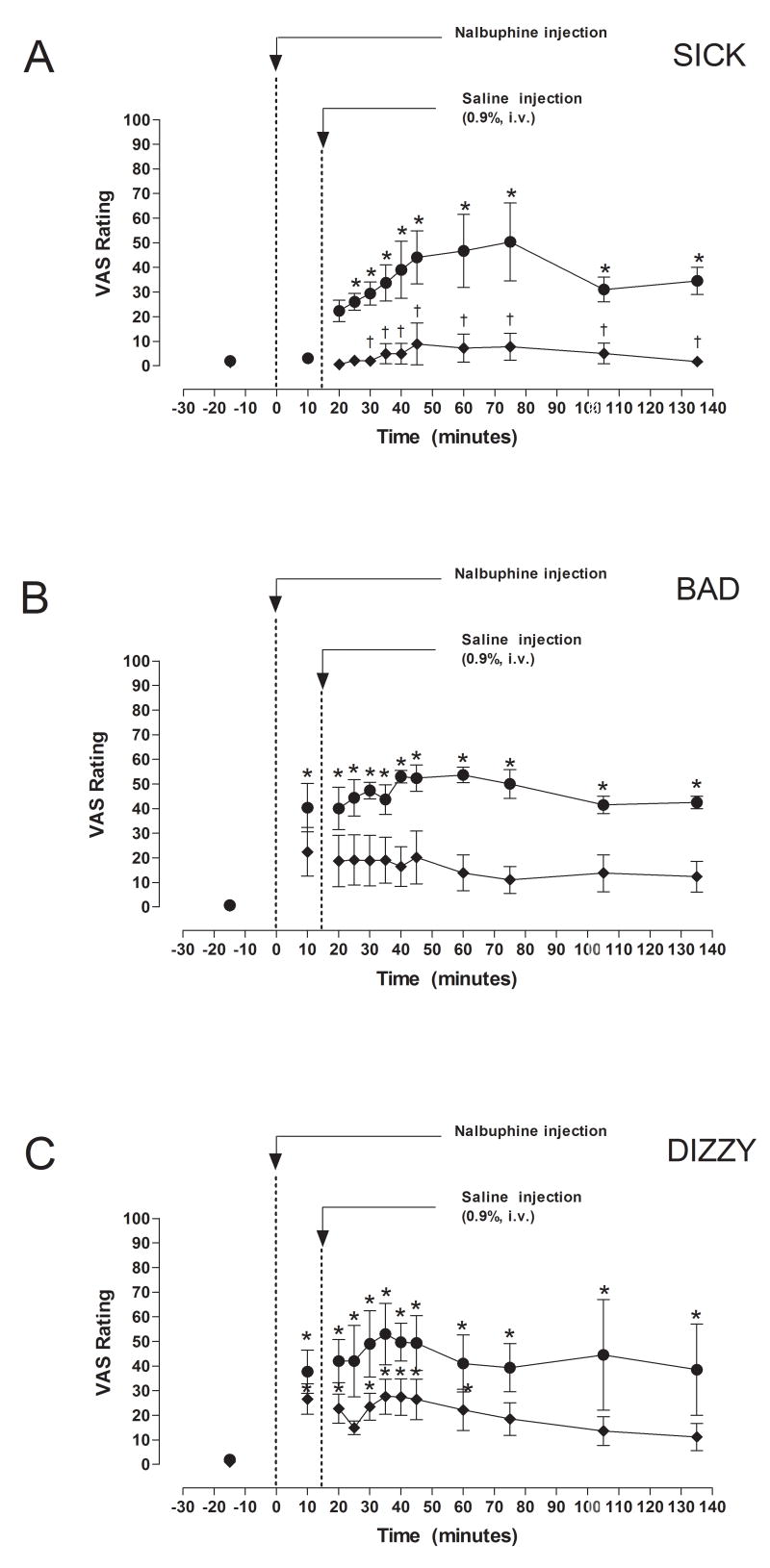
Effect of Nalbuphine on Subjective Responses (Figure 4)
Fig. 4.
VAS ratings of “Sick”, “Bad” and “Dizzy” after IV administration of 5 or 10 mg/kg nalbuphine. Significant changes from baseline are indicated by asterisks (*, P < 0.05). Times when VAS ratings differed between subjects who received 5 or 10 mg nalbuphine are indicated by daggers (†, P <0.05).
VAS measurements of “Sick”, “Bad” and “Dizzy” were collected before and after nalbuphine administration (Fig. 4). VAS ratings of “Sick” after 10 mg nalbuphine/70 kg were significantly higher (P < 0.05) than VAS ratings after 5 mg nalbuphine/70 kg at 30, 35, 40, 45, 60, 75, 105 and 135 min after IV nalbuphine. Subjects who received 10 mg nalbuphine/70 kg reported feeling “Sick” within 25 minutes after administration of the drug (P < 0.05), and continued to feel “Sick” at all time points for the next 110 minutes (Fig. 4). In contrast, subjects who received 5 mg nalbuphine/70 kg did not report feeling “Sick” during the study. Administration of 10 mg nalbuphine/70 kg also increased VAS ratings of “Bad” at all time points (P < 0.05), whereas no significant changes in VAS ratings of “Bad” were reported by subjects who received 5 mg nalbuphine/70 kg (Fig. 4). VAS ratings of “Bad” were not significantly different at any time points between subjects who received 5 or 10 mg nalbuphine/70 kg. Subjects who received 10 mg nalbuphine/70 kg reported feeling “Dizzy” at all time points after drug administration, whereas subjects who received 5 mg nalbuphine/70 kg reported feeling “Dizzy” only during the first 60 minutes (Fig. 4). VAS reports of “Dizzy” did not differ between subjects who received 5 or 10 mg nalbuphine/70 kg.
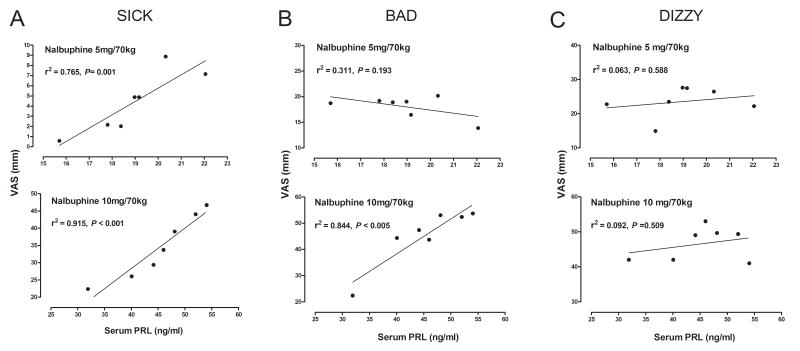
Correlation Between Serum PRL Levels and Subjective Effects
As shown earlier, serum PRL levels increased monotonically between 19 and 60 minutes after administration of both doses of nalbuphine (Fig. 2). Increases in VAS ratings of “Sick” were strongly correlated with serum PRL levels after 5 mg/70 kg nalbuphine (r2 = 0.765, P = 0.001) and 10 mg/70 kg nalbuphine (r2 = 0.915, P < 0.001). VAS ratings of “Bad” also correlated strongly (r2 = 0.844, P < 0.005) with serum PRL levels after 10 mg/70 kg nalbuphine but not after 5 mg/70 kg nalbuphine. No significant correlations were found between VAS ratings of “Dizzy” and serum PRL levels after either dose of nalbuphine (Fig. 5C)
Figure 5.
Discussion
This is the first clinical study to investigate the effects of nalbuphine on the HPA axis hormones and ratings of subjective effects in male cocaine abusers. Peak levels of nalbuphine were detected within 10 min after IV administration of 5 mg/70 kg and 10 mg/70 kg nalbuphine. One major finding of the present study was that a significant nalbuphine dose-dependent increase in serum PRL levels was detected within 20 min, and levels continued to increase until reaching peak values after 60 min. In our previous studies, using similar methodology, IV administration of the vehicle alone did not result in a significant change in PRL from the baseline levels (see Mendelson et al., 2003).
The observed increase in serum PRL levels after IV nalbuphine administration is in agreement with a study in healthy male and female volunteers, which showed that IM nalbuphine (0.15 mg/kg) increased plasma prolactin levels to peak levels that were 4-fold higher than baseline, within 1 hour after administration (Saarialho-Kere, 1988). These clinical data are also consistent with findings from a study of the effects of nalbuphine on prolactin in rhesus monkey (Butelman et al., 2002). Nalbuphine stimulated a dose-dependent increase in prolactin in a cumulative dosing procedure, and these effects were surmountably antagonized by the mixed action mu/kappa opioid antagonist nalmefene (Butelman et al., 2002).
In contrast to nalbuphine’s effects on PRL, there were no significant changes in ACTH or cortisol. This was surprising because ACTH and cortisol are also considered to be stress-reactive hormones. Moreover, baseline cortisol levels were in the range previously measured in cocaine abusers in whom cocaine stimulated a significant increase in ACTH and cortisol (Mendelson et al., 2002). Thus hypercortisolemia could not account for the lack of changes in ACTH and cortisol in the present study. The contrast between nalbuphine’s effects on PRL and ACTH and cortisol may reflect its actions as both a mu and a kappa agonist and these possibilities are discussed below.
VAS ratings of “Sick”, “Bad” and “Dizzy” were significantly higher in subjects who received 10 mg/70 kg nalbuphine than in those who received 5 mg/70 kg nalbuphine. Additionally, the negative effects of 10 mg nalbuphine in this sample were substantially greater than those previously reported in healthy volunteers (Walker et al., 2001; Zacny et al., 1997). Nalbuphine-induced increases in PRL were significantly correlated with VAS ratings of “Sick” and “Bad” after 10 mg/70 kg nalbuphine. The lower dose of nalbuphine was significantly correlated with “Sick”. These subjective ratings are consistent with the classification of PRL as a stress-reactive hormone (Marti and Armario, 1998). In our previous studies, no change from baseline in subjective effects was observed after vehicle administration alone (Mendelson et al., 2003).
Prolactin Regulation
As noted earlier, tuberoinfundibular dopaminergic neurons (TIDA) play a predominant role in control of PRL release (Ben-Jonathan, 1985; Ben-Jonathan and Hnasko, 2001). Continuous release of dopamine (DA) is ensured by its high rate of synthesis via hydroxylation of tyrosine to DOPA. Dopamine activity at D2 receptors tonically inhibits PRL release from anterior pituitary and maintain its low circulating levels (Ben-Jonathan, 1985). Opioid peptides of all three classes (mu-, kappa- and delta-) are known to interact with dopaminergic systems within median eminence. A number of neural systems and estradiol (E) (Ben-Jonathan and Hnasko, 2001) are thought to be involved in opioid-mediated hormone release, including noradrenergic (Carr and Gregg, 1995), cholinergic (Kaur, 2001), GABAergic (Kaur, 2001), serotonergic (Foresta et al., 1986), and dopaminergic systems (Butelman and Kreek, 2001; Schlussman et al., 2002).
Both mu and kappa opioid agonists stimulate prolactin release (Bart et al., 2003; Ellingboe et al., 1980; Kreek, 1978; Kreek et al., 1999), so it is likely that the nalbuphine-induced increases in serum prolactin levels observed in the present study involve interactions with both kappa and mu opioid receptors. The most likely mechanism for the effects of opioids on hormone release relates to TIDA, which consists of neurons with cell bodies in the arcuate and periventricular nuclei of the hypothalamus and axons that project into the median eminence of the hypothalamus. Dopamine released from these axons inhibit prolactin release by binding to dopamine D2 receptors located on pituitary lactotrophs (Ben-Jonathan, 1985; Ben-Jonathan and Hnasko, 2001; Yen and Jaffe, 1999). Thus, a decrease in dopamine levels would lead to increased PRL release. Indeed, preclinical studies have reported decreased extracellular dopamine levels in the nucleus accumbens and caudate putamen in response to kappa agonists such as salvinorin A (Zhang et al., 2005), U-69593 (Thompson et al., 2000), U-50488 (Donzanti et al., 1992), and R-8470 (Zhang et al., 2004). Other studies suggest that opioids may interfere with PRL regulation by activating mu receptors and causing hyperpolarization of dopamine neurons, which inhibits their spontaneous activity (Loose and Kelly, 1989; Loose et al., 1990). Although nalbuphine is generally regarded to be a mixed-action mu receptor antagonist and kappa agonist (Jaffe and Martin, 1990) recent in vitro studies suggest that nalbuphine is also an agonist at the mu receptor, with a variable efficacy to that of morphine (Emmerson et al., 1996; Gharagozlou et al., 2003). These data raise the possibility that nalbuphine administration may increase prolactin release through its mu agonist activity as well as its kappa agonist activity. Preclinical studies and clinical studies described below suggest that perturbations of the HPA axis reflected in PRL release are influenced by involvement of both kappa and mu opioid receptors.
Preclinical Studies of Prolactin Interactions with Kappa and Mu Opioids
In preclinical studies, increases in PRL levels in response to kappa opioid receptor agonists were reported in rats (Matton et al., 1991), sheep (Parrott and Goode, 1993) and rhesus monkeys (Butelman et al., 1999a, b, c, 2001, 2002, 2004). Similarly, PRL levels increased in response to mu agonists in rats (Koenig et al., 1984; Limonta et al., 1987; Matton et al., 1991; Pan and Teo, 1989; Shin et al., 1988; Simpkins et al., 1991; Spiegel et al., 1982), sheep (Parrott and Goode, 1993), and rhesus monkeys (Bowen et al., 2002; Gilbeau et al., 1985).
Clinical studies of Prolactin interactions with Kappa and Mu Opioids
Serum prolactin levels increased after administration of kappa opioid receptor-selective agonists dynorphin A1–13 (Bart et al., 2003; Kreek et al., 1999) and spiradoline (Ur et al., 1997) in normal healthy volunteers. Increases in serum prolactin levels have been observed after administration of a variety of mu opioid- selective agonists, including oxycodone (Saarialho-Kere et al., 1989), meptazinol (Kay et al., 1985), morphine (Delitala et al., 1983), dermorphin (Degli Uberti et al., 1983) in normal healthy volunteers. Heroin and methadone also stimulate prolactin release in opioid-dependent men (Bart et al., 2003; Ellingboe et al., 1980; Kreek, 1978). Furthermore, mixed action mu-kappa opioids such as buprenorphine and nalmefene also increase serum prolactin levels in humans (Bart et al., 2005; Rolandi et al., 1983). These data suggest that the nalbuphine-induced increases in serum prolactin levels observed in the present study may involve interactions of the drug with both kappa and mu opioid receptors.
Preclinical Studies of ACTH and Cortisol interactions with Mu and Kappa Opioids
The mu-selective agonists loperamide (Giagnoni et al., 1982) and morphine increased ACTH and corticosterone in rats possibly via a CRF-independent mechanism; e.g. altering expression of c-Fos in the paraventricular nucleus (PVN): site of CRF neurons known to initiate ACTH secretion (Buckingham and Cooper, 1984, 1986; Coventry et al., 2001; Giagnoni et al., 1982; Houshvar et al., 2001a, b; Suemaru et al., 1986; Zhou et al., 1999). However, in other studies, loperamide and the mu-selective agonists fentanyl decreased basal levels of ACTH as well CRH-induced ACTH and cortisol in rat cultures (Auernhammer et al., 1993). An increase in CRF and ACTH after kappa-receptor selective agonist MR 2034 administration was observed in rats, possibly via both CRH-dependent and independent mechanism (Colagero et al., 1996).
Clinical Studies of ACTH and Cortisol Interactions with Mu and Kappa Opioids
An unexpected finding was that plasma ACTH and serum cortisol did not change significantly after either dose of nalbuphine. The literature with respect to nalbuphine’s effects on HPA and HPG axes hormones via modulation of mu- and kappa-opioid receptors is controversial and possibly varies among species (Calogero et al., 1989; Pfeiffer et al., 1986). The loperamide and fentanyl decreased basal levels of ACTH as well CRH-induced ACTH and cortisol release in humans (Auernhammer et al., 1992). The kappa-agonist MR 2033 inhibited ACTH and cortisol secretion at “stereotypic, non-classical opioid receptors” in a naloxone reversible manner (Pfeiffer et al., 1986). However, there was an unexpected large increase in ACTH and cortisol secretion following administration of the same kappa agonist (Pfeiffer et al., 1986). Furthermore, a dose-dependent stimulation of cortisol release by kappa-selective agonist spiradoline was reported in healthy human volunteers (Ur et al., 1997).
The mu opioid antagonists naloxone and naltrexonee increase plasma levels of ACTH and cortisol in humans (Hernandez-Avila et al., 2002; King et al., 2002; Mendelson et al., 1986 Mendelson et al., 1991; Teoh et al., 1988; Wand et al., 1999a, b). Additionally, the mixed mu/kappa opioid buprenorphine, significantly attenuated cortisol levels in normal human volunteers (Pende et al., 1986). However, the mixed action mu/kappa antagonist nalmefene produced significant increases in ACTH and cortisol in healthy volunteers (Schluger et al., 1998). Nalmefene induced increases in ACTH were greater than those observed after naloxone (Schluger et al., 1998). Considering that nalbuphine may act as both a mu agonist and a mu antagonist in addition to its kappa agonist activity, these data suggest that the interaction of these oppositional forces may account for nalbuphine’s dual kappa-like and mu-like effects on ACTH and cortisol.
In summary, the significant increase in levels of the anterior pituitary hormone PRL following administration of the mixed mu/kappa drug nalbuphine is consistent with the effect of mu agonists and kappa agonists alone. Interestingly, this mixed action did not increase ACTH and cortisol even though subjects reported feeling “Sick”. These VAS ratings were significantly correlated with increases in PRL. Because mu agonists alone inhibit ACTH and cortisol release, these findings suggest that the mu-agonist component of nalbuphine may have prevented stress-related and kappa agonist-induced stimulation of ACTH and cortisol. The dissociation between the responses of these “stress” labile hormones to nalbuphine was unexpected.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auernhammer CJ, Renner U, Muller OA, Stalla J, Stalla GK. Loperamide inhibits corticotrophic cell function by a naloxone-insensitive mechanism in the rat in vitro. Neuroendocrinology. 1993;57:1019–1027. doi: 10.1159/000126466. [DOI] [PubMed] [Google Scholar]
- Auernhammer CJ, Riepl RL, Schopohl J, Lehnert P, Muller OA, Stalla GK. In man the mu-opiate agonist loperamide specifically inhibits ACTH secretion induced by the cholecystokinin-like peptide ceruletide. Neuroendocrinology. 1994;60:16–22. doi: 10.1159/000126715. [DOI] [PubMed] [Google Scholar]
- Auernhammer CJ, Stalla GK, Lange M, Pfeiffer A, Muller OA. Effects of loperamide on the human hypothalamo-pituitary-adrenal axis in vivo and in vitro. J Clin Endocrinol Metab. 1992;75:552–557. doi: 10.1210/jcem.75.2.1322429. [DOI] [PubMed] [Google Scholar]
- Bart G, Borg L, Schluger JH, Green M, Ho A, Kreek MJ. Suppressed prolactin response to dynorphin A1–13 in methadone-maintained versus control subjects. J Pharmacol Exp Ther. 2003;306:581–587. doi: 10.1124/jpet.103.050682. [DOI] [PubMed] [Google Scholar]
- Bart G, Schluger JH, Borg L, Ho A, Bidlack JM, Kreek MJ. Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity? Neuropsychopharmacology. 2005;30:2254–2262. doi: 10.1038/sj.npp.1300811. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rabii J. Inhibition of suckling-induced prolactin release by mu- and kappa-opioid antagonists. Brain Res. 1991;567:224–230. doi: 10.1016/0006-8993(91)90799-2. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N. Dopamine: A prolactin-inhibiting hormone. Endocrine Rev. 1985;6:564–589. doi: 10.1210/edrv-6-4-564. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocrine Rev. 2001;22:724–763. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- Bigelow GE, Brady JV, Griffiths RR, Stitzer ML, Ator NA, Higgins ST, Liebson IA, Lucas SE. Progress report from the Division of Behavioral Biology, the Johns Hopkins University School of Medicine. NIDA Res Monogr. 1984;55:66–75. [PubMed] [Google Scholar]
- Bowen CA, Negus SS, Kelly M, Mello NK. The effects of heroin on prolactin levels in male rhesus monkeys: use of cumulative-dosing procedures. Psychoneuroendocrinology. 2002;27:319–336. doi: 10.1016/s0306-4530(01)00053-1. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, Mello NK. Effects of mixed-action kappa-mu opioids on cocaine self-administration and cocaine discrimination by rhesus monkeys. Neuropsychopharmacology. 2003;28:1125–1139. doi: 10.1038/sj.npp.1300105. [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinology. 1984;38:411–417. doi: 10.1159/000123927. [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Effects of naloxone on hypothalamo-pituitary-adrenocortical activity in the rat. Neuroendocrinology. 1986;42:421–426. doi: 10.1159/000124481. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ball JW, Kreek MJ. Comparison of the discriminative and neuroendocrine effects of centrally penetrating kappa-opioid agonists in rhesus monkeys. Psychopharmacology (Berl) 2002;164:115–120. doi: 10.1007/s00213-002-1195-y. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ball JW, Kreek MJ. Peripheral selectivity and apparent efficacy of dynorphins: comparison to non-peptidic kappa-opioid agonists in rhesus monkeys. Psychoneuroendocrinology. 2004;29:307–326. doi: 10.1016/s0306-4530(03)00030-1. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek M. Apparent efficacy of kappa-opioid receptor ligands on serum prolactin levels in rhesus monkeys. Eur J Pharmacol. 1999a;383:305–309. doi: 10.1016/s0014-2999(99)00640-8. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek MJ. Effects of E-2078, a stable dynorphin A(1–8) analog, on sedation and serum prolactin levels in rhesus monkeys. Psychopharmacology (Berl) 1999b;147:73–80. doi: 10.1007/s002130051144. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Perez A, Kreek MJ. Effects of systemically administered dynorphin A(1–17) in rhesus monkeys. J Pharmacol Exp Ther. 1999c;290:678–686. [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Traynor JR, Vivian JA, Kreek MJ, Woods JH. GR89,696: a potent kappa-opioid agonist with subtype selectivity in rhesus monkeys. J Pharmacol Exp Ther. 2001;298:1049–1059. [PubMed] [Google Scholar]
- Butelman ER, Kreek MJ. kappa-Opioid receptor agonist-induced prolactin release in primates is blocked by dopamine D(2)-like receptor agonists. Eur J Pharmacol. 2001;423:243–249. doi: 10.1016/s0014-2999(01)01121-9. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Gallucci WT, Kling MA, Chrousos GP, Gold PW. Cocaine stimulates rat hypothalamic corticotropin-releasing hormone secretion in vitro. Brain Research. 1989;505:7–11. doi: 10.1016/0006-8993(89)90109-1. [DOI] [PubMed] [Google Scholar]
- Carr JA, Gregg KJ. Opioid peptide inhibition of endogenous norepinephrine release from the A2 noradrenergic cell group in vitro. Neuropeptides. 1995;28:219–225. doi: 10.1016/0143-4179(95)90025-x. [DOI] [PubMed] [Google Scholar]
- Cohen SE, Ratner EF, Kreitzman TR, Archer JH, Mignano LR. Nalbuphine is better than naloxone for treatment of side effects after epidural morphine. Anesth Analg. 1992;75:747–752. [PubMed] [Google Scholar]
- Colagero AE, Scaccianoce S, Burrello N, Nicolai R, Muscolo LA, Kling MA, Angelucci L, D’Agata R. The kappa-opioid receptor agonist MR-2034 stimulates the rat hypothalamic-pituitary-adrenal axis: studies in vivo and in vitro. Neuroendocrinology. 1996;8:579–585. [PubMed] [Google Scholar]
- Coventry TL, Jessop DS, Finnn DP, Crabb MD, Kinoshita H, Harbguz MS. Endomorphins and activation of the hypothalamo-pituitary-adrenal axis. J Endocrinology. 2001;169:185–193. doi: 10.1677/joe.0.1690185. [DOI] [PubMed] [Google Scholar]
- Degli Uberti EC, Trasforini G, Salvadori S, Margutti A, Tomatis R, Bianconi M, Rotola C, Pansini R. Responses of plasma renin activity, aldosterone, adrenocorticotropin, and cortisol to dermorphin, a new synthetic potent opiate-like peptide, in man. Clin Endocrinol Metab. 1983;57:1179–1185. doi: 10.1210/jcem-57-6-1179. [DOI] [PubMed] [Google Scholar]
- Delitala G, Grossman A, Besser GM. The participation of hypothalamic dopamine in morphine-induced prolactin release in man. Clin Endocrinol (Oxf) 1983;19:437–444. doi: 10.1111/j.1365-2265.1983.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Donzanti BA, Althaus JS, Payson MM, Von Voigtlander PF. Kappa agonist-induced reduction in dopamine release: site of action and tolerance. Res Commun Chem Pathol Pharmacol. 1992;78:193–210. [PubMed] [Google Scholar]
- Ellingboe J, Mendelson JH, Kuehnle JC. Effects of heroin and naltrexone on plasma prolactin levels in man. Pharmacol Biochem Behav. 1980;12:163–165. doi: 10.1016/0091-3057(80)90431-1. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther. 1996;278:1121–1127. [PubMed] [Google Scholar]
- Errick JK, Heel RC. Nalbuphine. A preliminary review of its pharmacological properties and therapeutic efficacy. Drugs. 1983;26:191–211. doi: 10.2165/00003495-198326030-00002. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Christiansen I, Levin FR, Fischman MW. Effects of single and multiple intravenous cocaine injections in humans maintained on methadone. J Pharmacol Exp Ther. 1995;275:38–47. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. The cardiovascular and subjective effects of intravenous cocaine and morphine combinations in humans. J Pharmacol Exp Ther. 1992;261:623–632. [PubMed] [Google Scholar]
- Foresta C, Mioni R, Scandellari C. Evidence for serotoninergic system involvement in opioid control of luteinizing hormone secretion in man. Clin Endocrinol (Oxf) 1986;25:573–578. doi: 10.1111/j.1365-2265.1986.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Gharagozlou P, Demirci H, David Clark J, Lameh J. Activity of opioid ligands in cells expressing cloned mu opioid receptors. BMC Pharmacol. 2003;3:1. doi: 10.1186/1471-2210-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagnoni G, Parolaro D, Sala M, Marabini L, Senini R, Gori E. Increase of plasma corticosterone induced by loperamide in rats. Eur J Pharmacol. 1982;79:101–104. doi: 10.1016/0014-2999(82)90579-9. [DOI] [PubMed] [Google Scholar]
- Gilbeau PM, Almirez RG, Holaday JW, Smith CG. Opioid effects on plasma concentrations of luteinizing hormone and prolactin in the adult male rhesus monkey. J Clin Endocrinol Metab. 1985;60:299–305. doi: 10.1210/jcem-60-2-299. [DOI] [PubMed] [Google Scholar]
- Grossman A. Brain opiates and neuroendocrine function. Clin Endocrinol Metab. 1983;12:725–746. doi: 10.1016/s0300-595x(83)80062-0. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Oncken C, Van Kirk J, Wand G, Kranzler HR. Adrenocorticotropin and cortisol responses to a naloxone challenge and risk of alcoholism. Biol Psychiatry. 2002;51:652–658. doi: 10.1016/s0006-3223(01)01334-8. [DOI] [PubMed] [Google Scholar]
- Hoehe M, Duka T, Doenicke A. Human studies on the mu opiate receptor agonist fentanyl: neuroendocrine and behavioral responses. Psychoneuroendocrinology. 1988;13:397–408. doi: 10.1016/0306-4530(88)90046-7. [DOI] [PubMed] [Google Scholar]
- Houshvar H, Cooper ZD, Woods JH. Paradoxical effects of chronic morphine treatment on the temperature and pituitary-adrenal responses to acute restraint stress: a chronic stress paradigm. Neuroendocrinology. 2001a;13:862–874. doi: 10.1046/j.1365-2826.2001.00713.x. [DOI] [PubMed] [Google Scholar]
- Houshvar H, Galigniana MD, Pratt WB, Woods JH. Differential responsivity of the hypothalamic-pituitary-adrenal axis to glucocorticoid negative-feedback and corticotropin releasing hormone in rats undergoing morphine withdrawal: possible mechanisms involved in facilitated and attenuated stress responses. Neuroendocrinology. 2001b;13:875–886. doi: 10.1046/j.1365-2826.2001.00714.x. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Martin WR. Opioid Analgesics and Antagonists. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. New York: Pergamon Press; 1990. pp. 485–521. [Google Scholar]
- Jamidar HA, Crooks SW, Adgey AA. Nalbuphine versus diamorphine early in the course of suspected myocardial infarction. Eur Heart J. 1987;8:597–602. doi: 10.1093/oxfordjournals.eurheartj.a062328. [DOI] [PubMed] [Google Scholar]
- Kaur G. Role of cholinergic and GABAergic neurotransmission in the opioids-mediated GnRH release mechanism of EBP-primed OVX rats. Mol Cell Biochem. 2001;219:13–19. doi: 10.1023/a:1011027717543. [DOI] [PubMed] [Google Scholar]
- Kay NH, Allen MC, Bullingham RE, Baldwin D, McQuay RJ, Moore HA, Price RK, Sear JW. Influence of meptazinol on metabolic and hormonal responses following major surgery. A comparison with morphine. Anaesthesia. 1985;40:223–228. doi: 10.1111/j.1365-2044.1985.tb10746.x. [DOI] [PubMed] [Google Scholar]
- King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenocortical (HPA) axis response and biotransformation of oral naltrexone: preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 2002;26:778–788. doi: 10.1016/S0893-133X(01)00416-X. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Mayfield MA, McCann SM, Krulich L. Differential role of the opioid mu and delta receptors in the activation of prolactin (PRL) and growth hormone (GH) secretion by morphine in the male rat. Life Sci. 1984;34:1829–1837. doi: 10.1016/0024-3205(84)90676-3. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Medical complications in methadone patients. In: Kissin B, Lowinson J, Millman R, editors. Recent Developments in Chemotherapy of Narcotic Addiction. New York: Ann. Acad. Sci.; 1978. pp. 110–134. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Schluger J, Borg L, Gunduz M, Ho A. Dynorphin A1–13 causes elevation of serum levels of prolactin through an opioid receptor mechanism in humans: gender differences and implications for modulation of dopaminergic tone in the treatment of addictions. J Pharmacol Exp Ther. 1999;288:260–269. [PubMed] [Google Scholar]
- Lake CL, Duckworth EN, DiFazio CA, Magruder MR. Cardiorespiratory effects of nalbuphine and morphine premedication in adult cardiac surgical patients. Acta Anaesthesiol Scand. 1984;28:305–309. doi: 10.1111/j.1399-6576.1984.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Limonta P, Maggi R, Dondi D, Martini L, Piva F. Gonadal steroid modulation of brain opioid systems. J Steroid Biochem. 1987;27:691–698. doi: 10.1016/0022-4731(87)90138-5. [DOI] [PubMed] [Google Scholar]
- Loose MD, Kelly MJ. Opioid inhibition of spontaneously active neurons of the rat arcuate nucleus in vitro. Brain Res Bull. 1989;22:819–823. doi: 10.1016/0361-9230(89)90024-5. [DOI] [PubMed] [Google Scholar]
- Loose MD, Ronnekleiv OK, Kelly MJ. Membrane properties and response to opioids of identified dopamine neurons in the guinea pig hypothalamus. J Neurosci. 1990;10:3627–3634. doi: 10.1523/JNEUROSCI.10-11-03627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Brady JV, Griffiths RR. Comparison of opioid self-injection and disruption of schedule-controlled performance in the baboon. J Pharmacol Exp Ther. 1986;238:924–931. [PubMed] [Google Scholar]
- Manzanares J, Wagner EJ, Moore KE, Lookingland KJ. Kappa opioid receptor-mediated regulation of prolactin and alpha-melanocyte-stimulating hormone secretion in male and female rats. Life Sci. 1993;53:795–801. doi: 10.1016/0024-3205(93)90501-s. [DOI] [PubMed] [Google Scholar]
- Marti O, Armario A. Anterior pituitary response to stress: time-related changes and adaptation. Int J Dev Neurosci. 1998;16:241–260. doi: 10.1016/s0736-5748(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Matton A, Buydens P, Finne E, Govaerts J, Vanhaelst L. Analysis of the receptor specificity of tolerance induction in stress versus opioid-related prolactin secretion in rats. J Endocrinol. 1991;128:281–285. doi: 10.1677/joe.0.1280281. [DOI] [PubMed] [Google Scholar]
- McBride AJ, Williamson K, Petersen T. Three cases of nalbuphine hydrochloride dependence associated with anabolic steroid use. Br J Sports Med. 1996;30:69–70. doi: 10.1136/bjsm.30.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Kamien JB, Lukas SE, Drieze J, Mendelson JH. The effects of nalbuphine and butorphanol treatment on cocaine and food self-administration by rhesus monkey. Neuropsychopharmacology. 1993;8:45–55. doi: 10.1038/npp.1993.6. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Sholar MB, Jaszyna-Gasior M, Goletiani NV, Siegel AJ. Effects of the mixed mu/kappa opioid nalbuphine on cocaine-induced changes in subjective and cardiovascular responses in men. Neuropsychopharmacology. 2005;30:618–632. doi: 10.1038/sj.npp.1300631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Interactions between kappa opioid agonists and cocaine: Preclinical studies. In: Glick SD, Maisonneuve IM, editors. The Archer Conference on Drug Abuse: New Medications. New York: New York Academy of Sciences; 2000. pp. 104–132. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Cristofaro P, Skupny A, Ellingboe J. Use of naltrexone as a provocative test for hypothalamic-pituitary hormone function. Pharmacol Biochem Behav. 1986;24:309–313. doi: 10.1016/0091-3057(86)90356-4. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Mutschler N, Halpern J. Temporal concordance of cocaine effects on mood states and neuroendocrine hormones. Psychoneuroendocrinology. 2002;27:71–82. doi: 10.1016/s0306-4530(01)00036-1. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Teoh SK, Ellingboe J. Use of naltrexone for the diagnosis and treatment of reproductive hormone disorders in women. In: Harris LS, editor. Problems of Drug Dependence 1990. Washington, D.C.: U.S. Government Printing Office; 1991. pp. 161–167. [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Mutschler NH, Jaszyna-Gasior M, Goletiani NV, Siegel AJ, Mello NK. Effects of intravenous cocaine and cigarette smoking on luteinizing hormone, testosterone and prolactin in men. J Pharmacol Exp Ther. 2003;307:339–348. doi: 10.1124/jpet.103.052928. [DOI] [PubMed] [Google Scholar]
- Miller RR. Evaluation of nalbuphine hydrochloride. Am J Hosp Pharm. 1980;37:942–949. [PubMed] [Google Scholar]
- Pan JT, Teo KL. Fentanyl stimulates prolactin release through mu-opiate receptors, but not the serotonergic system. Endocrinology. 1989;125:1863–1869. doi: 10.1210/endo-125-4-1863. [DOI] [PubMed] [Google Scholar]
- Parrott RF, Goode JA. Central effects of naloxone and selected opioid agonists on cortisol and prolactin secretion in non-stressed sheep. Gen Pharmacol. 1993;24:101–103. doi: 10.1016/0306-3623(93)90017-r. [DOI] [PubMed] [Google Scholar]
- PDR, editor. Physicians Desk Reference. 57. Montvale, NJ: Thomson PDR; 2003. [Google Scholar]
- PDR. Physicians Desk Reference. 58. Montvale, NJ: Thomson PDR; 2005. [Google Scholar]
- Pende A, Musso NR, Montaldi ML, Pastorino G, Arzese M, Devilla L. Evaluation of the effects induced by four opiate drugs with diffefrent affinities to opioid receptor subtypes, on anterior pituitary LH, TSH, PRL and GH secretion and on cortisol secretion in normal men. Biomed Pharmacother. 1986;40:178–182. [PubMed] [Google Scholar]
- Pfeiffer A, Herz A, Loriaux DL, Pfeiffer DG. Central kappa- and mu-opiate receptors mediate ACTH-release in rats. Endocrinology. 1985;116:2688–2690. doi: 10.1210/endo-116-6-2688. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Knepel W, Braun S, Meyer HD, Lohmann H, Brantl V. Effects of a kappa-opioid agonist on adrenocorticotropic and diuretic function in man. Horm Metab Res. 1986;18:842–848. doi: 10.1055/s-2007-1012453. [DOI] [PubMed] [Google Scholar]
- Pick CG, Paul D, Pasternak GW. Nalbuphine, a mixed kappa 1 and kappa 3 analgesic in mice. J Pharmacol Exp Ther. 1992;262:1044–1050. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Effects of Agonist-Antagonist Opioids in Humans Trained in a Hydromorphone/Not Hydromorphone Discrimination. J Pharmacol Exp Ther. 2000;295:114–124. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA. Antagonist effects of nalbuphine in opioid-dependent humans. J Pharmacol Exp Ther. 1989;248:929–937. [PubMed] [Google Scholar]
- Preston KL, Jasinski DR. Abuse liability studies of opioid agonist-antagonists in humans. Drug Alcohol Depend. 1991;28:49–82. doi: 10.1016/0376-8716(91)90053-2. [DOI] [PubMed] [Google Scholar]
- Rolandi E, Marabini A, Franceschini R, Messina V, Bongera P, Barreca T. Changes in pituitary secretion induced by an agonist-antagonist opioid drug, buprenorphine. Acta Endocrinol (Copenh) 1983;104:257–260. doi: 10.1530/acta.0.1040257. [DOI] [PubMed] [Google Scholar]
- Romagnoli A, Keats AS. Ceiling effect for respiratory depression by nalbuphine. Clin Pharmacol Ther. 1980;27:478–485. doi: 10.1038/clpt.1980.67. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. Psychomotor, respiratory and neuroendocrinological effects of nalbuphine and haloperidol, alone and in combination, in healthy subjects. Br J Clin Pharmacol. 1988;26:79–87. doi: 10.1111/j.1365-2125.1988.tb03367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U, Mattila MJ, Seppala T. Psychomotor, respiratory and neuroendocrinological effects of a mu-opioid receptor agonist (oxycodone) in healthy volunteers. Pharmacol Toxicol. 1989;65:252–257. doi: 10.1111/j.1600-0773.1989.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Ho A, Borg L, Porter M, Maniar S, Gunduz M, Perret G, King A, Kreek MJ. Nalmefene causes greater hypothalamic-pituitary-adrenal axis activation than naloxone in normal volunteers: implications for the treatment of alcoholism. Alcohol Clin Exp Res. 1998;22:1430–1436. doi: 10.1111/j.1530-0277.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Nyberg F, Kreek MJ. The effects of drug abuse on the stress responsive hypothalamic-pituitary-adrenal axis and the dopaminergic and endogenous opioid systems. Acta Psychiatr Scand Suppl. 2002:121–124. doi: 10.1034/j.1600-0447.106.s412.26.x. [DOI] [PubMed] [Google Scholar]
- Shin SH, Obonsawin MC, Van Vugt DA, Baby N, Jhamandas K. Morphine can stimulate prolactin release independent of a dopaminergic mechanism. Can J Physiol Pharmacol. 1988;66:1381–1385. doi: 10.1139/y88-226. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Swager D, Millard WJ. Evaluation of the sites of opioid influence on anterior pituitary hormone secretion using a quaternary opiate antagonist. Neuroendocrinology. 1991;54:384–390. doi: 10.1159/000125918. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Kourides IA, Pasternak GW. Prolactin and growth hormone release by morphine in the rat: different receptor mechanisms. Science. 1982;217:745–747. doi: 10.1126/science.6285470. [DOI] [PubMed] [Google Scholar]
- Suemaru S, Hashimoto K, Ota A. Effect of morphine on hypothalamic corticotropin-releasing factor (CRF) and pituitary-adrenocortical activity. Endocrinol Jpn. 1986;33:441–448. doi: 10.1507/endocrj1954.33.441. [DOI] [PubMed] [Google Scholar]
- Teoh SK, Mendelson JH, Mello NK, Skupny A. Alcohol effects on naltrexone-induced stimulation of pituitary, adrenal and gonadal hormones during the early follicular phase of the menstrual cycle. J Clin Endocrinol Metab. 1988;66:1181–1186. doi: 10.1210/jcem-66-6-1181. [DOI] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ur E, Wright DM, Bouloux PM, Grossman A. The effects of spiradoline (U-62066E), a kappa-opioid receptor agonist, on neuroendocrine function in man. Br J Pharmacol. 1997;120:781–784. doi: 10.1038/sj.bjp.0700971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DJ, Zacny JP, Galva KE, Lichtor JL. Subjective psychomotor, and physiological effects of cumulative doses of mixed-action opioids in healthy volunteers. Psychopharmacology. 2001;155:362–371. doi: 10.1007/s002130100723. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of κ-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001b;299:147–158. [PubMed] [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner J. The effects of naltrexone on response to i.v. cocaine, hydromorphone and their combination in humans. J Pharmacol Exp ther. 1996;279:524–538. [PubMed] [Google Scholar]
- Wand GS, Mangold D, Ali M. Adrenocorticotropin responses to naloxone in sons of alcohol-dependent men. J Clin Endocrinol Metab. 1999b;84:61–68. doi: 10.1210/jcem.84.1.5373. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, Ali M, Giggey P. Adrenocortical responses and family history of alcoholism. Alcohol Clin Exp Res. 1999a;23:1185–1190. [PubMed] [Google Scholar]
- Williams KL, Ko MC, Rice KC, Woods JH. Effect of opioid receptor antagonists on hypothalamic-pituitary-adrenal activity in rhesus monkeys. Psychoneuroendocrinology. 2003;28:513–528. doi: 10.1016/s0306-4530(02)00037-9. [DOI] [PubMed] [Google Scholar]
- Wines JD, Jr, Gruber AJ, Pope HG, Jr, Lukas SE. Nalbuphine hydrochloride dependence in anabolic steroid users. Am J Addict. 1999;8:161–164. doi: 10.1080/105504999305965. [DOI] [PubMed] [Google Scholar]
- Woollard M, Jones T, Pitt K, Vetter N. Hitting them where it hurts? Low dose nalbuphine therapy. Emerg Med J. 2002;19:565–570. doi: 10.1136/emj.19.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen SSC, Jaffe RB. Prolactin in human reproduction. In: Yen SSC, Jaffe RB, Barbieri RL, editors. Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Philadelphia: W. B. Saunders Co.; 1999. pp. 257–283. [Google Scholar]
- Zacny JP, Conley K, Galinkin J. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J Pharmacol Exp Ther. 1997;282:1187–1197. [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the kappa opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004;173:146–152. doi: 10.1007/s00213-003-1716-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Maggos CE, Wang XM, Han JS, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenal activity and pro-opiomelanocortin mRNA levels in the hypothalamus and pituitary of the rat are differentially modulated by acute intermittent morphine with or without water restriction stress. J Endocrinol. 1999;163:261–267. doi: 10.1677/joe.0.1630261. [DOI] [PubMed] [Google Scholar]
- Zhu J, Luo LY, Li JG, Chen C, Liu-Chen LY. Activation of the cloned human kappa opioid receptor by agonists enhances [35S]GTPgammaS binding to membranes: determination of potencies and efficacies of ligands. J Pharmacol Exp Ther. 1997;282:676–684. [PubMed] [Google Scholar]