Abstract
Hypoglossal motoneuron output to the genioglossus muscle contributes to upper airway patency. Serotonin (5HT) plays an important role in regulating hypoglossal motoneuron excitability via serotonin 2A receptors (5HT2A). The purpose of this study was to investigate whether there are age-associated changes in 5HT2A receptor expression in the hypoglossal nucleus of male and female rats. The brains of young, middle-aged and old F344 rats were sectioned, reacted immunocytochemically for the presence of 5HT2A receptor, and the staining density quantified. The estrus stage of female rats was determined and circulating sex hormone levels measured and correlated with 5HT2A levels. The results show that there was significantly greater 5HT2A receptor immunoreactivity in the hypoglossal nucleus of female than of male rats. With increasing age, there was an increase in 5HT2A receptor immunoreactivity in the hypoglossal nucleus of female rats, whereas no age-associated changes were observed in male rats. Previous studies have shown a reduction in 5HT-dependent respiratory plasticity and an age-associated decrease in 5HT in the hypoglossal nucleus in male but not female rats. Data from the present study suggest that aging male rats fail to compensate adequately for reduced 5HT in the hypoglossal nucleus by upregulating the expression of the 5HT2A receptor.
Keywords: Control of breathing, Respiratory motoneurons, Estrogen, Progesterone, Sex hormones
1. Introduction
Serotonin (5-hydroxytryptamine or 5-HT) plays a pivotal role in the control of breathing (for review, see Bonham, 2005; Bianchi et al., 1995; McCrimmon et al., 1995; Richter et al., 2003). Caudal raphe neurons are activated during hypoxia and release 5HT in the vicinity of respiratory premotor and motoneurons (Erickson and Millhorn, 1994; Teppema et al., 1997). Serotonin has an excitatory effect on respiratory motoneurons, mediated principally by 5HT2 receptors (Kubin et al., 1992; Lindsay and Feldman, 1993; Arita et al., 1995; Jelev et al., 2001; Fenik and Veasey, 2003; Brandes et al., 2006).
Hypoglossal motoneurons innervate muscles of the tongue that contribute to upper airway patency during inspiration (Remmers et al., 1978; Miki et al., 1989; Horner, 1996; Saboisky et al., 2006). Serotonergic input to the hypoglossal nucleus originates in neurons in the nucleus raphe pallidus and obscurus and the parapyramidal region (Manaker and Tischler, 1993). Studies have shown that 5HT modulation of hypoglossal motoneuron output is mediated by one or more postsynaptic 5HT2 receptor subtypes (Schwarzacher et al., 2002; Volgin et al., 2003). The most prominent 5HT2 receptor subtype in hypoglossal motoneurons is 2A (Zhan et al., 2002), and application of selective 2A antagonists to the hypoglossal nucleus reduces hypoglossal nerve respiratory activity by >60% (Fenik and Veasey, 2003). As serotonergic neurons are tonically active during wakefulness, and decrease their firing rate during sleep (Jacobs and Azmitia, 1992), withdrawal of 5HT excitatory drive during sleep is thought to reduce upper airway patency and contribute to sleep apnea (Kubin et al., 1996)
There are age-associated changes in 5HT modulation of respiratory plasticity in both male and female rats (Zabka et al., 2001a,b). Long term facilitation (LTF) is a 5HT-dependent plasticity in hypoglossal and phrenic motor output in response to intermittent hypoxia (for review see Mitchell and Johnson, 2003). The 5HT2 receptor is critical to this form of respiratory plasticity as LTF can be abolished by ketanserin, a 5HT2 receptor antagonist (Kinkead et al., 1998; Ling et al., 2001; Fuller et al., 2001). LTF is significantly reduced in middle-aged male rats by comparison with young rats (Zabka et al., 2001a). In contrast, female rats show enhanced LTF in middle-age (Zabka et al., 2001b), suggesting that this form of respiratory plasticity is sexually dimorphic.
Sex hormones may play a role in regulating respiratory plasticity. LTF in respiratory motoneurons in response to intermittent hypoxia, and 5HT levels in respiratory motor nuclei fluctuate with the estrus cycle (Zabka et al., 2001b; Behan et al., 2003). In geriatric female rats that have ceased to cycle regularly, there is a decline in hypoglossal and phrenic LTF by comparison with middle-aged rats (Zabka et al., 2003), and gonadectomized young male rats also show reduced LTF in hypoglossal motor output (Behan et al., 2003).
Altered serotonergic modulation of respiratory motoneurons with age in male or female rats could result from a number of different mechanisms including a decrease in serotonergic innervation of respiratory nuclei and/or changes in receptor expression. Previous studies in our laboratory have shown that there are sex differences in 5HT levels in the hypoglossal nucleus: more in females than males (Behan et al., 2003). We hypothesized that female rats would have greater 5HT2A receptor expression in the hypoglossal nucleus than male rats. With increasing age there is a decrease in serotonergic terminals and altered synaptic bouton morphology in the hypoglossal nucleus of male rats (Behan and Brownfield, 1999; Behan et al., 2002), whereas female rats do not show a decrease (M. Behan, unpublished observations). Additionally, middle-aged male but not female rats have reduced hypoglossal LTF by comparison with young rats (Zabka et al., 2001 a, b). Thus, we hypothesized that 5HT2A receptor levels in the hypoglossal nucleus of male and female rats would be differentially affected by age. In the present study we measured 5HT2A receptor immunoreactivity in the hypoglossal nucleus of young, middle-aged and old male and female rats. Additionally, we tested the hypothesis that circulating estrogen and progesterone levels correlate with 5HT2A receptor immunoreactivity in the hypoglossal nucleus in female rats.
2. Methods
2.1. Animals and Tissue Preparation
All procedures were performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Wisconsin School of Veterinary Medicine. Three age groups were studied: young (3 months), middle-aged (14 months), and old (>20 months). A total of 12 male rats (4 young, 4 middle-aged, 4 old) and 15 female F344 rats (6 young [3 estrus, 3 diestrus], 6 middle-aged [3 estrus, 3 diestrus], 3 old) were used in the present study. Estrus cycle stage was determined by vaginal lavage and light microscopy (Hebel and Stromberg, 1984). Young and middle-aged female rats were followed through at least two estrus cycles. Old female rats are generally acyclic. They were followed for approximately one week to confirm their lack of cycling, and establish their estrus cycle stage.
Rats were anesthetized with sodium pentobarbital (50mg/ml i.p.). Anesthetized rats were transcardially perfused with 200ml of heparinized saline (10,000 units/liter) followed by 400ml of 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M sodium phosphate buffer (PB) (pH 7.4). Brains were removed and postfixed for 1 hour at 4°C, then cryoprotected for 24–36 h at 4°C with 20% sucrose and 5% glycerol in 0.1M phosphate buffer. Sections were cut in the horizontal plane with a freezing microtome at a thickness of 50μm. Sections that were not reacted immediately were stored in 0.1M phosphate buffer containing 0.02% sodium azide at 4°C.
2.2. Immunocytochemical Staining
All sections through the hypoglossal nucleus were reacted for the presence of 5HT2A receptor. Free floating sections were incubated in 3% normal goat serum (NGS) in 0.1M sodium phosphate buffer (PB) for 30 minutes. Sections were then reacted with antiserum to 5HT2A receptor (gift from M. Brownfield; Brownfield et al., 1998) in 1% NGS (1:500) for 18 hours at 4°C. The same lot of 5HT2A antibody was used in all immunocytochemical reactions. Sections were washed in 0.3% Triton-X in 0.1M PB (2×15 mins) and incubated in 3% NGS in PB at 4°C. Sections were incubated in biotinylated goat anti-rabbit IgG (1:200, Vector Laboratories, Burlingame, CA) in 1% NGS with 0.75% Triton-X for 1 hour. Sections were washed (2×15 mins) and incubated with ABC complex (Vectastain Elite Kit, Vector Laboratories) for 1 hour. Sections were washed with 0.1 M PBS (5×5 min), and reacted with 0.04% DAB in 0.1 M PB with 0.003% hydrogen peroxide and 0.1% nickel ammonium sulfate. Sections were washed, mounted and cover slipped.
2.3. Data Analysis
Digital photographs (16 bit; 65,536 grey levels) of brain sections were taken using a SPOT camera (Diagnostic Instruments; optical resolution 1600 × 1200 pixels; 7.4μm square pixels) mounted on a Nikon Omniphot microscope, and quantitative image analysis was performed with ImagePro Plus software. All images were obtained under identical bright field illumination. The paired hypoglossal nuclei could be seen clearly in each horizontal section (Fig. 1). Staining in the hypoglossal nucleus was darker than in the overlying dorsal vagal motor nucleus. This, combined with localization of the central canal and fourth ventricle, allowed the dorsal vagal motor nucleus to be identified and excluded from the analysis. Two photographs were taken of each section: one of the paired hypoglossal nuclei and one of a region with homogeneous light 5HT2A staining. A rectangular sample area was defined (62,500 μm2) and optical density, which measures the darkness of each pixel, was measured in rostral, middle and caudal samples bilaterally (Fig. 1). To control for background labeling, optical density was measured in a region of homogeneous light staining, a fiber tract that could be easily identified in each horizontal section. This value was subtracted from each measurement of 5HT2A density in that section (Fig. 1). Data were analyzed using either a one-way or two-way repeated measures ANOVA (SigmaStat, Version 2.0, Jaendel, San Rafael, CA). Differences were considered significant at P < 0.05.
Figure 1.
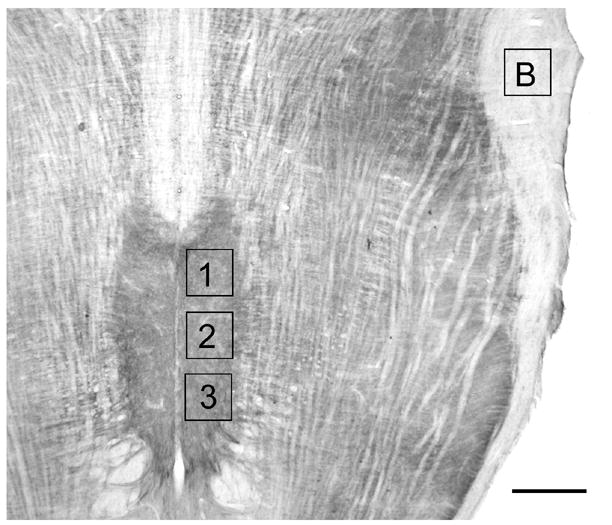
Photomicrograph of a horizontal section through the medulla stained for 5HT2A immunoreactivity. Rostral (1), middle (2) and caudal (3) sample areas are shown together with the location of the sample area analyzed for background label (B). Scale bar = 750 μm.
2.4. Measurement of Sex Hormone Levels
Prior to perfusion, blood was drawn from the abdominal aorta in female rats for measurement of sex hormone levels. Blood samples were centrifuged, and serum was immediately frozen and stored at −80°C. After collection of all serum samples, 17β estradiol (the most potent mammalian estrogenic hormone) and progesterone levels were analyzed by ELISA (IBL, Hamburg, Germany; 17β estradiol, Cat. # RE50421, sensitivity 4.6 – 3000 pg/ml; progesterone, Cat. #RE52231, sensitivity 0.05 – 36 ng/ml). Serum levels of estradiol and progesterone and the progesterone-to-estradiol (P/E) ratio of individual rats were related to the magnitude of 5HT2A immunoreactivity via linear regression (SigmaStat, Version 2.0, Jaendel, San Rafael, CA). A variable was considered to significantly contribute to the model if P< 0.05.
3. Results
3.1. 5HT2A Immunoreactivity in Male and Female Rats
Dense 5HT2A immunoreactivity was present throughout the hypoglossal nucleus by comparison with the surrounding neuropil (Fig. 1). When considered as a group, female rats had significantly more 5HT2A immunoreactivity in the hypoglossal nucleus by comparison with male rats (P<0.001) (Fig. 2). This sex difference was present in the middle-aged (P = 0.009) and old groups of rats (P = 0.001), but not in the young group (P = 0.068). Sex differences were present in rostral (P = 0.022), middle (P = 0.001) and caudal (P < 0.001) regions of the hypoglossal nucleus, and in dorsal (P = 0.004) and ventral (P = 0.003) subdivisions.
Figure 2.
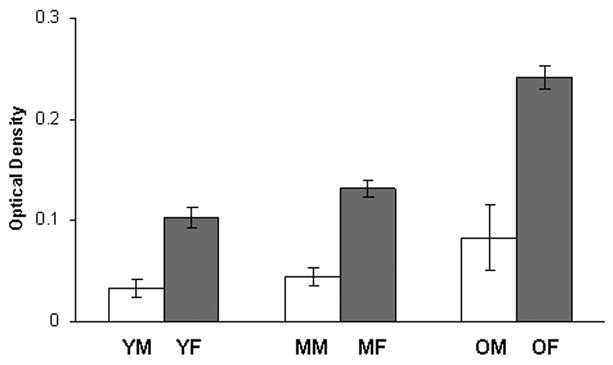
Quantitative analysis of 5HT2A receptor immunoreactivity in the hypoglossal nucleus of young, middle-aged and old male and female rats. Receptor level was measured by optical density (OD), averaged within each rat and then across rats in each group. Middle-aged and old females has significantly more 5HT2A receptor immunoreactivity than males. Old females also has significantly more 5HT2A receptor immunoreactivity than young (P=0.001) and middle-aged (P=0.002) females. There were no significant differences between young, middle-aged and old male rats. YM, young male, n=4; MM, middle-aged male, n=4; OM, old male, n=4; YF, young female, n=6; MF, middle-aged female, n=6; OF, old female, n=3. Values are means ± S.E.
3.2. Age-related changes in 5HT2A in Male and Female Rats
There was a statistically significant increase in 5HT2A immunoreactivity in the hypoglossal nucleus of old female rats by comparison with young (P <0.001) and middle-aged females (P = 0.002) (Figs. 2, 3). Significant differences were found in all three regions: rostral (old vs. young, P = 0.001; old vs. middle-aged, P = 0.016), middle (old vs. young, P = 0.001; old vs. middle-aged, P = 0.004) and caudal (old vs. young, P = 0.001; old vs. middle-aged, P = 0.001). Significant differences were also found in dorsal (old vs. young, P = 0.006; old vs. middle-aged, P = 0.034) and ventral (old vs. young, P = 0.004; old vs. middle-aged, P = 0.036) subdivisions of the hypoglossal nucleus. In young and middle-aged female rats, 5HT2A levels varied with the estrus cycle: greater in diestrus than in estrus, although this was not statistically significant (Fig. 4). This trend was also seen in rostral, middle and caudal regions of the hypoglossal nucleus. In contrast to female rats, there was no significant effect of age on 5HT2A immunoreactivity in any region of the hypoglossal nucleus in male rats (Fig. 2).
Figure 3.
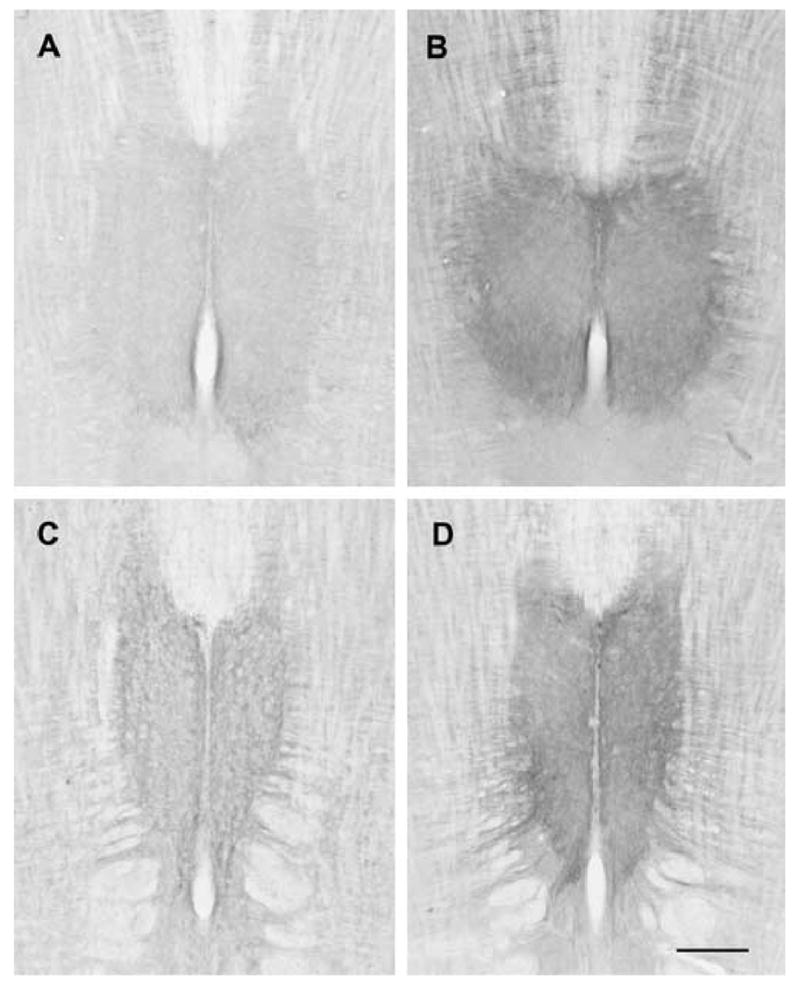
Photomicrographs of horizontal sections through the hypoglossal nucleus of young and old female rats stained for 5HT2A immunoreactivity. A. Young female, dorsal section. B. Old female, dorsal section. C. Young female, ventral section. D. Old female, ventral section. Sections from young and old rats were reacted at the same time. Images were captured using identical illumination parameters; no further adjustments were made. Scale bar = 750 μm.
Figure 4.
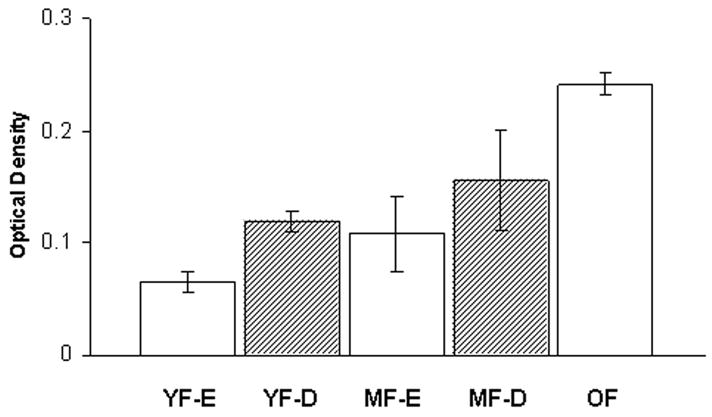
Quantitative analysis of 5HT2A receptor immunoreactivity in the hypoglossal nucleus of female rats in estrus and diestrus. Receptor level was measured by optical density (OD), averaged within each rat and then across rats in each group. Although there is a trend towards an increase in diestrus, this was not significant. YF-E, young female in estrus, n=3; YF-D, young female in diestrus, n=3; MF-E, middle-aged female in estrus,, n=3; MF-D, middle-aged female in diestrus, n=3; O, old female, n=3. Values are means ± S.E.
3.3. Sex hormone levels and 5HT2A Immunoreactivity
Levels of 17 β estradiol (pg/ml) and progesterone (ng/ml) were correlated with 5HT2A immunoreactivity in the hypoglossal nucleus in young, middle-aged and old female rats (Fig. 5A). Linear regression showed no statistically significant relationship between 5HT2A receptor immunoreactivity and sex hormone levels in female rats (n = 15). However, when 5HT2A receptor immunoreactivity in the ventral half of the hypoglossal nucleus was analyzed in young and middle-aged female rats (n = 12; this region contains motoneurons that innervate protrusor tongue muscles; Aldes, 1995), there was a weak but significant relationship between 5HT2A and P/E (R2 = 0.384; P = 0.032). The relationship between 5HT2A receptor immunoreactivity in the ventral half of the hypoglossal nucleus and P/E in rats in diestrus (young and middle-aged, n = 6) was also not statistically significant (R2 = 0.647; P = 0.054) (Fig. 5B). The relationship between 5HT2A receptor immunoreactivity in the ventral half of the hypoglossal nucleus and P/E in rats in estrus (young and middle-aged, n = 6) was not significant (R2 = 0.603; P = 0.070) (Fig. 5C).
Figure 5.
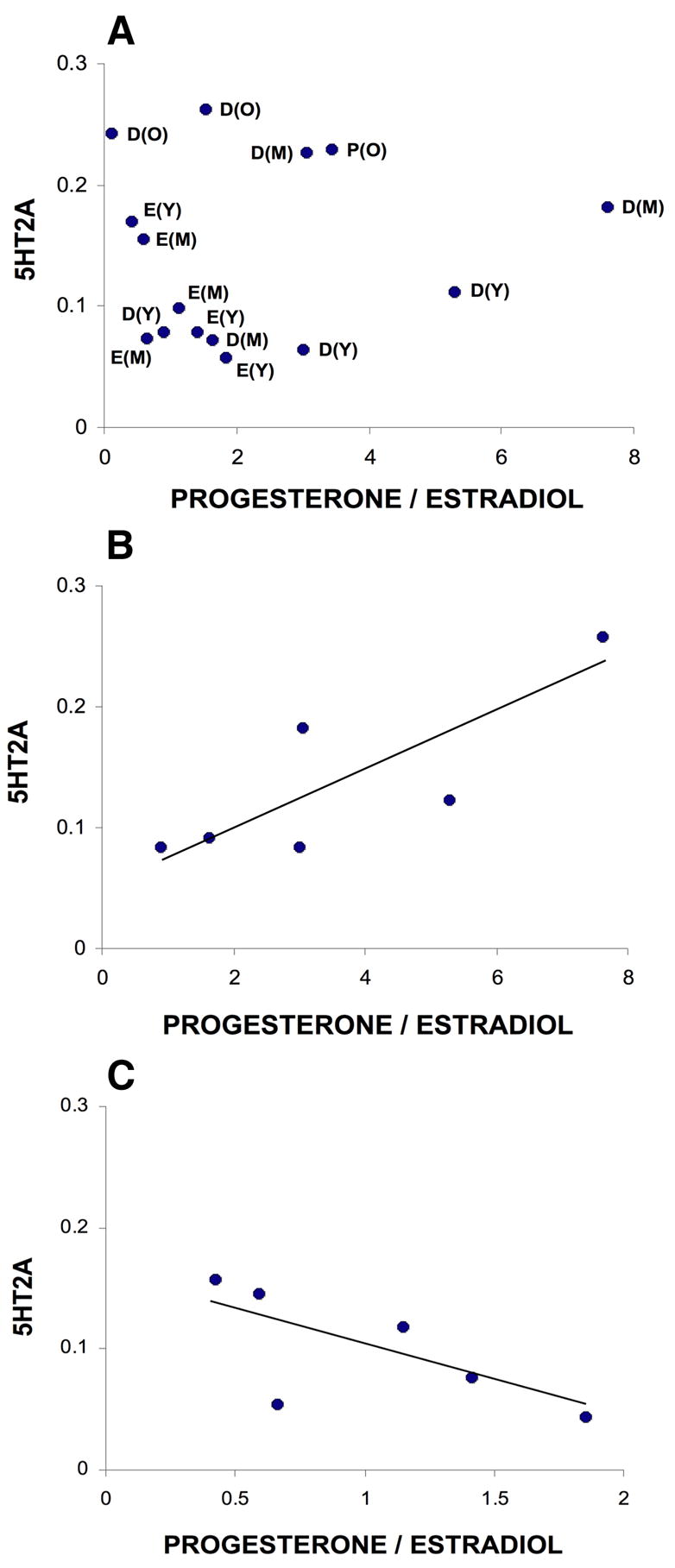
Relationship between 5HT2A immunoreactivity in the hypoglossal nucleus and sex hormone levels. A. The magnitude of 5HT2A immunoreactivity (optical density) in the hypoglossal nucleus is plotted against progesterone/estradiol for all15 female rats. The age and estrus stage of each rat is indicated. B. Relationship between the magnitude of 5HT2A immunoreactivity in the ventral half of the hypoglossal nucleus and progesterone/estradiol in rats in young and middle-aged rats in diestrus (n = 6; R2 = 0.647; P = 0.054). C. Relationship between the magnitude of 5HT2A immunoreactivity in the ventral half of the hypoglossal nucleus and progesterone/estradiol in young and middle-aged rats in estrus (n = 6; R2 = 0.603; P = 0.070). D, diestrus; E, estrus; P, proestrus; Y, young; M, middle aged; O, old.
4. Discussion
The main findings of this study are: (1) there is sexual dimorphism in 5HT2A receptor immunoreactivity in the rat hypoglossal nucleus; (2) there is an age-associated increase in 5HT2A receptor immunoreactivity in the hypoglossal nucleus of female but not male rats.
4.1. Sexual Dimorphism in the Serotonergic System
Several studies have reported greater circulating levels of 5HT in women by comparison with men (Ashcroft et al., 1964; Wirz-Justice et al., 1977; Ortiz et al., 1988). In the mammalian nervous system sex differences have been reported in the morphology of 5HT neurons and in 5HT synthesis, turnover and receptor binding (Carlsson and Carlsson, 1988; Nishizawa et al., 1997; Rubinow et al., 1998; Zhang et al., 1999; Cordero et al., 2001). Most studies have focused on the cerebral cortex, hippocampus, hypothalamus and dorsal raphe nuclei with a view to understanding the role of 5HT in depression, learning and memory. However, sex differences are also present in the medulla. Previously we reported that levels of 5HT in the hypoglossal nucleus of female rats, as measured by ELISA, were double that of male rats (Behan et al., 2003). Although the number of serotonergic neurons in the medullary raphe that project to the hypoglossal nucleus is similar in male and female rats (Barker and Behan, 2006), synaptic terminal density is greater in the hypoglossal nucleus of female rats (M. Behan, unpublished observations). In the present study we show that 5HT2A receptor immunoreactivity in the hypoglossal nucleus is greater in female than in male rats (Fig. 2). Thus, by several measures there appears to be a more robust serotonergic innervation of the hypoglossal nucleus in female than in male rats. As 5HT has an excitatory effect on upper airway motoneurons mediated by 5HT2A and 5HT2C receptor subtypes (Fenik and Veasey, 2003), sex differences in 5HT input to, or 5HT2A receptor expression in hypoglossal motoneurons may contribute to the susceptibility of males to breathing disorders such as obstructive sleep apnea (OSA). Nonetheless, recent data shows that there is a negligible serotonergic drive to hypoglossal motoneurons during natural sleep in rats (Sood et al., 2005). The prevalence of OSA is greater in men than in women (Young et al., 1993; Bixler et al., 1998, 2001). After menopause, estrogen and progesterone appear to have a neuroprotective effect on sleep disordered breathing in women (Bixler et al., 2001; Young et al., 2003; Shahar et al., 2003).
4.2. Estrogen and Progesterone in the Serotonergic System
The female rat estrus cycle is 4–5 days in duration and consists of 4 stages: proestrus (~12 hrs), estrus (~12 hrs), metestrus (~21 hrs), diestrus (~57 hrs) (Freeman 1994). Levels of plasma estradiol are low in estrus, higher in diestrus. There are two peaks in plasma progesterone levels, a lower peak in metestrus and a higher peak late in proestrus. During normal hormonal fluctuations, there are variations in 5HT synthesis, release, reuptake and turnover (Biegon et al., 1980; Bethea et al., 2000; Lu et al., 2003; Pekins-Thompson et al., 1998) and in 5HT receptor expression (Biegon et al., 1980; Moses et al., 2000; Birzniece et al., 2002; Kugaya et al., 2003) in rats and primates. Additionally, treatment with estrogen or progesterone has been shown to alter 5HT1A mRNA levels in the dorsal raphe of non-human primates (Pecins-Thompson and Bethea, 1999).
Previously we found that 5HT levels in the rat hypoglossal nucleus were higher in diestrus than in estrus (Behan et al., 2003). Consistent with this, in the present study there was a trend towards higher levels of 5HT2A receptor immunoreactivity in the hypoglossal nucleus in diestrus by comparison with estrus in young and middle-aged rats (Fig. 4), suggesting that receptors can upregulate very rapidly. Correlations between sex hormone levels (P/E) and 5HT2A receptor levels in rats in estrus or diestrus are weak (Fig. 5B, C), and a larger data set is needed to clarify these potential relationships. Whether estradiol and progesterone influence 5HT synthesis, release and/or reuptake in the hypoglossal nucleus and thereby regulate 5HT2A receptor expression, or whether they act directly on hypoglossal motoneurons is not known. Estrogen receptors are present on both hypoglossal and medullary raphe neurons, and it likely that these neurons also express progesterone receptors (Behan and Thomas, 2005; Mitra et al., 2003; Bethea, 1993).
Serotonin is known to play a critical role in respiratory plasticity (for review, see Mitchell and Johnson, 2003). Consistent with the finding that hypoglossal 5HT and 5HT2A receptor levels are greater in diestrus than in estrus, hypoglossal LTF also varies with the estrus cycle: greater in diestrus than in estrus (Zabka et al., 2001b). Thus, respiratory plasticity in hypoglossal motor output in female rats in response to intermittent hypoxia (as measured by LTF) appears to be influenced by circulating sex hormone levels via the serotonergic system. Although the physiological relevance of LTF is not yet clear, it may play a protective role, particularly during sleep when serotonergic activity is low. In a recent study in rats, ventilatory LTF in response to intermittent hypoxia was observed during non-REM sleep, but not during wakefulness (Nakamura et al., 2006). Ventilatory instability during sleep, resulting in intermittent hypoxia, could activate serotonergic neurons to upregulate breathing and upper airway tone via LTF of hypoglossal motoneuron output and an increase in upper airway muscle activity (Suratt et al., 1988; Babcock and Badr, 1998; Morelli et al., 2004). Interestingly, upper airway resistance during sleep varies with the menstrual cycle: lower in the luteal phase than in the follicular phase (Driver et al., 2005). Thus, it is possible that serotonergic modulation of upper airway motoneurons might also vary with the menstrual/estrus cycle.
The relative influence of estrogen and progesterone on 5HT2A receptor expression in the hypoglossal nucleus is not clear. Our data suggest that 5HT2A receptor immunoreactivity is correlated with circulating hormone levels especially in the ventral half of the hypoglossal nucleus where motoneurons that innervate protrusor muscles are located (Aldes, 1995), but the nature of this relationship differs between diestrus and estrus (Fig. 5B, C). Hypoglossal LTF also differs in diestrus and estrus in young and middle-aged female rats (Zabka et al., 2001b), and in a recent study of young and geriatric female rats, there was a significant correlation between hypoglossal LTF and the progesterone-to-estradiol ratio (Zabka et al., 2003). Ultimately, the relative influence of estradiol and progesterone in regulating upper airway patency may depend on a critical ratio of these two hormones that changes across the estrus cycle as well as throughout life.
4.3. Aging and the Serotonergic System
Age-associated changes have been described in serotonergic input to the hypoglossal nucleus in male rats including a reduction in the number of 5HT terminals and an increase in axons with aberrant morphology (Behan and Brownfield, 1999; Behan et al., 2002). Hypoglossal LTF also diminishes with aging in male rats (Zabka et al., 2001a; 2005), suggesting that sertonergic function is impaired. An age-associated reduction in 5HT in the hypoglossal nucleus without a compensatory 5HT2A receptor upregulation could contribute to the reduction in hypoglossal LTF in aging male rats. Whether sex hormones might directly influence medullary raphe or hypoglossal neurons in male rats is not yet known, although androgen and estrogen receptors are present on hypoglossal neurons (Behan and Thomas, 2005).
4.4. Conclusion
Several lines of evidence suggest that there is a relationship between circulating sex hormone levels and the serotonergic system. Dynamic changes that occur in sex hormone levels can influence a 5HT-dependent form of respiratory plasticity in hypoglossal motoneurons, and may also have an effect on levels of 5HT and the 5HT2A receptor in the hypoglossal nucleus. With increasing age and a decline in hormone levels in male rats, or the loss of regular cycles in female rats, the relationship between the serotonergic system and sex hormones may be weakened, and respiratory challenges that require adaptive responses may result in functional impairment, especially in male rats.
Acknowledgments
Supported by NIA-AG18760 to M. Behan. B.S. Seebart and R.J. Stoffel were supported by Summer Research Fellowships in Veterinary Medicine from Merck-Merial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldes LD. Subcompartmental organization of the ventral (protrusor) compartment in the hypoglossal nucleus of the rat. J Comp Neurol. 1995;353:89–108. doi: 10.1002/cne.903530109. [DOI] [PubMed] [Google Scholar]
- Arita H, Ichilawa K, Sakamoto M. Serotonergic cells in nucleus raphe pallidus provide tonic drive to posterior cricoarytenoid motoneurons via 5-hydroxytryptamine receptors in cats. Neurosci Lett. 1995;197:113–116. doi: 10.1016/0304-3940(95)11907-e. [DOI] [PubMed] [Google Scholar]
- Ashcroft GW, Crawford TB, Binns JK, Macdougall EJ. Estimation of 5-hydroxytryptamine in human blood. Clin Chim Acta. 1964;9:364–369. doi: 10.1016/0009-8981(64)90027-0. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Badr S. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–716. [PubMed] [Google Scholar]
- Barker JR, Behan M. Sexual dimorphism in serotonergic input to the hypoglossal nucleus. FASEB J. 2006;20:A782-c. [Google Scholar]
- Behan M, Brownfield MS. Age-related changes in serotonin in the hypoglossal nucleus of rat: Implications for sleep-disordered breathing. Neurosci Lett. 1999;267:133–136. doi: 10.1016/s0304-3940(99)00337-7. [DOI] [PubMed] [Google Scholar]
- Behan M, Thomas CF. Sex hormone receptors are expressed in identified respiratory motoneurons in male and female rats. Neuroscience. 2005;130:725–734. doi: 10.1016/j.neuroscience.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Mitchell GS. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol. 2002;131:65–77. doi: 10.1016/s1569-9048(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Thomas CF, Mitchell GS. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol. 2003;136:249–263. doi: 10.1016/s1569-9048(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Bethea CL. Colocalization of progestin receptors with serotonin in raphe neurons of macaque. Neuroendocrinology. 1993;57:1–6. doi: 10.1159/000126334. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Gundlah C, Mirkes SJ. Ovarian steroid action in the serotonin neural system of macaques. Novartis Found Symp. 2000;230:112–33. doi: 10.1002/0470870818.ch9. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Biegon A, Bercovitz H, Samuel D. Serotonin receptor concentration during the estrous cycle of the rat. Brain Res. 1980;187:221–225. doi: 10.1016/0006-8993(80)90509-0. [DOI] [PubMed] [Google Scholar]
- Birzniece V, Backstrom T, Johansson IM, Lindblad C, Lundgren P, Lofgren M, Olsson T, Ragagnin G, Taube M, Turkmen S, Wahlstrom G, Wang MD, Wihlback AC, Zhu D. Neuroactive steroid effects on cognitive functions with a focus on the serotonin and GABA systems. Brain Res Brain Res Rev. 2006;51:212–239. doi: 10.1016/j.brainresrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin H, Have TT, Rein J, Vela-Beuno A, Kales A. Prevalence of sleep-disordered breathing in women. Am J Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- Bonham AC. Neurotransmitters in the CNS control of breathing. Respir Physiol. 1995;101:219–230. doi: 10.1016/0034-5687(95)00045-f. [DOI] [PubMed] [Google Scholar]
- Brandes IF, Zuperku EJ, Stucke AG, Jakovcevic D, Hopp FA, Stuth EA. Serotonergic modulation of inspiratory hypoglossal motoneurons in decerebrate dogs. J Neurophysiol. 2006;95:3449–3459. doi: 10.1152/jn.00823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownfield MS, Yracheta J, Chu F, Lorenz D, Diaz A. Functional chemical neuroanatomy of serotonergic neurons and their targets: Antibody production and immunocytochemistry (IHC) for 5HT, its precursor (5HTP) and metabolite (5HIAA), biosynthetic enzyme (TPH), transporter (SERT), and three receptors (5HT2A, 5HT5A, and 5HT7) In: Martin GR, Eglen RM, Hoyer D, Hamblin MW, Yocca F, editors. Advances in Serotonin Receptor Research. Vol. 861. New York: Acad Sci; 1998. pp. 232–233. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. In vivo evidence for a greater brain tryptophan hydroxylase capacity in female than in male rats. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:345–349. doi: 10.1007/BF00172108. [DOI] [PubMed] [Google Scholar]
- Cordero ME, Rodriguez A, Torres R, Valenzuela CY. Human raphe magnus nucleus: a Golgi-Cox study with emphasis on sex differences. Brain Res Dev Brain Res. 2001;131:85–92. doi: 10.1016/s0165-3806(01)00266-8. [DOI] [PubMed] [Google Scholar]
- Driver HS, McLean H, Kumar DV, Farr N, Day AG, Fitzpatrick MF. The influence of the menstrual cycle on upper airway resistance and breathing during sleep. Sleep. 2005;28:449–456. doi: 10.1093/sleep/28.4.449. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 613–658. [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Hebel R, Stromberg MW. Anatomy and Embryology of the Laboratory Rat. BioMed Verlag; Worthsee, Germany: 1986. p. 231. [Google Scholar]
- Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–853. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neurosci Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Reignier C, Pack AI, Davies RO. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced, REM sleep-like atonia. Sleep. 1996;19:187–195. [PubMed] [Google Scholar]
- Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Seibyl JP, Innis RB. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry. 2003;160:1522–1524. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurons by serotonin. J Physiol Lond. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques. Mol Psychiatry. 2003;8:353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ. Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Mitchell GS, Dekin MS. Glutamate, GABA, and serotonin in ventilatory control. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. 1995. pp. 151–218. [Google Scholar]
- Miki H, Hida W, Shindoh C, Kikuchi Y, Chonan T, Taguchi O, Inoue H, Takishima T. Effects of electrical stimulation of the genioglossus on upper airway resistance in anesthetized dogs. Am Rev Respir Dis. 1989;140:1279–1284. doi: 10.1164/ajrccm/140.5.1279. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morelli C, Badr MS, Mateika JH. Ventilatory responses to carbon dioxide at low and high levels of oxygen are elevated after episodic hypoxia in men compared with women. J Appl Physiol. 2004;97:1673–1680. doi: 10.1152/japplphysiol.00541.2004. [DOI] [PubMed] [Google Scholar]
- Moses EL, Drevets WC, Smith G, Mathis CA, Kalro BN, Butters MA, Leondires MP, Greer PJ, Lopresti B, Loucks TL, Berga SL. Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: a PET study. Biol Psychiatry. 2000;48:854–860. doi: 10.1016/s0006-3223(00)00967-7. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Wenninger JM, Olson EB, Bisgard GE, Mitchell GS. Ventilatory long term facilitation following intermittent hypoxia is state-dependent in rats. J Physiol Sci. 2006;56:S75. doi: 10.1152/japplphysiol.90778.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, Blier P, Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Artigas F, Gelpi E. Serotonergic status in human blood. Life Sci. 1988;43:983–990. doi: 10.1016/0024-3205(88)90543-7. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Brown NA, Bethea CL. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Brain Res Mol Brain Res. 1998;53:120–129. doi: 10.1016/s0169-328x(97)00286-6. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience. 1999;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003;9:542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44:839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol. 2006;95:2213–2221. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Gunther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience. 2002;115:1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, O'Connor GT, Rapoport DM, Robbins JA. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–1347. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am Rev Respir Dis. 1988;137:889–894. doi: 10.1164/ajrccm/137.4.889. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C. Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol. 1997;388:169–190. doi: 10.1002/(sici)1096-9861(19971117)388:2<169::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Volgin DV, Fay R, Kubin L. Postnatal development of serotonin 1B, 2 A and 2C receptors in brainstem motoneurons. Eur J Neurosci. 2003;17:1179–1188. doi: 10.1046/j.1460-9568.2003.02545.x. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Lichtsteiner M, Feer H. Diurnal and seasonal variations in human platelet serotonin in man. J Neural Transm. 1977;41:7–15. doi: 10.1007/BF01252961. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol. 2001a;531.2:509–514. doi: 10.1111/j.1469-7793.2001.0509i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Time dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl Physiol. 2001b;91:2831–2838. doi: 10.1152/jappl.2001.91.6.2831. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Olson EB, Jr, Behan M. Chronic intermittent hypoxia enhances respiratory long term facilitation in geriatric female rats. J Appl Physiol. 2003;95:2614–2623. doi: 10.1152/japplphysiol.00476.2003. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M. Ageing and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol. 2005;563:557–568. doi: 10.1113/jphysiol.2004.077511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]
- Zhan G, Shaheen F, Mackiewicz M, Fenik P, Veasey SC. Single cell laser dissection with molecular beacon polymerase chain reaction identifies 2A as the predominant serotonin receptor subtype in hypoglossal motoneurons. Neuroscience. 2002;113:145–154. doi: 10.1016/s0306-4522(02)00137-9. [DOI] [PubMed] [Google Scholar]


