Abstract
Molecular studies have shown several peculiarities in the regulatory mechanisms of gene expression in trypanosomatids. Protein coding genes are organized in long polycistronic units that seem to be constitutively transcribed. Therefore, post-transcriptional regulation of gene expression is considered to be the main point for control of transcript abundance and functionality. Here we describe the characterization of a 17 kDa RNA-binding protein from Trypanosoma cruzi (TcRBP19) containing an RNA recognition motive (RRM). This protein is coded by a single copy gene located in a high molecular weight chromosome of T. cruzi. Orthologous genes are present in the TriTryp genomes. TcRBP19 shows target selectivity since among the different homoribopolymers it preferentially binds polyC. TcRBP19 is a low expression protein only barely detected at the amastigote stage localizing in a diffuse pattern in the cytoplasm.
Keywords: Kinetoplastida, Trypanosoma cruzi, RNA binding proteins, RRM protein, TcRBP19
Trypanosoma cruzi, a protozoan parasite of the order Kinetoplastida, is the causative agent of Chagas' disease affecting several million people along South and Central America and Mexico. During its life cycle, T. cruzi deals with abrupt environmental changes as it interacts with an insect and a mammalian host. Consequently, several proteins have to be tightly regulated to allow the rapid adaptation that is essential for the parasite survival.
Kinetoplastida constitute a very early branch in eukaryotic evolution presenting several remarkable deviations from standard eukaryotic paradigms. Although transcription is polycistronic individual genes can exhibit completely different regulation (Teixeira 1998). There are no canonical promoters identified yet and there is no evidence for specific or regulated transcription initiation of protein coding genes by RNA polymerase II. Indeed, open reading frames investigated so far seem to be constitutively transcribed. The processing of the long polycistronic transcripts into monocistronic units is achieved by trans-splicing and polyadenylation in a coupled reaction (LeBowitz et al., 1993). Given these peculiarities, the regulation of gene expression occurs predominantly at the post-transcriptional level, and undoubtedly, RNA binding proteins (RBP) play a major role in this process.
The characterization of particular RBPs and the study of their molecular mechanisms would provide important insights in the understanding of the mechanisms of gene expression regulation in T. cruzi. Few RNA-binding proteins involved in mRNA-turnover control have been identified so far (D'Orso et al., 2003). Particular factors such as a polyA binding protein (Batista et al., 1994), a spliced leader RNA binding protein (Xu et al., 2001), Pumilio proteins (Dallagiovanna et al., 2005, Caro et al., 2006) and CCCH-type Zn-finger-containing proteins (Espinosa et al., 2003, Morking et al., 2004, Caro et al., 2005) have been reported in T. cruzi. Likewise, an RRM (RNA Recognition Motif) protein family implicated in mRNA abundance through the specific binding to U rich elements has been described (D'Orso and Frasch, 2001, D'Orso and Frasch, 2002, D'Orso et al., 2003, De Gaudenzi et al., 2003).
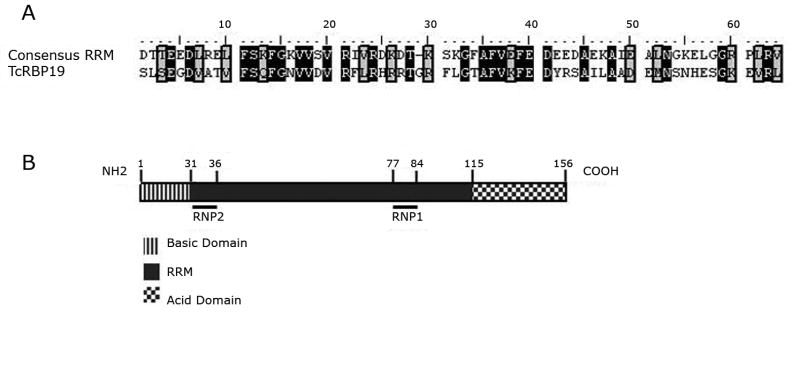
During our investigation on the pteridine reductase gene (Robello et al., 1997), RT-PCR assays done using total RNA amplified with a spliced leader based primer (5′-GCTATTATTGATACAGTTTCTG-3′) and a specific antisense oligonucleotide rendered different bands that were excised from the gel, cloned into pGEM-T vector (PROMEGA) and sequenced. A clone with an insert of 373bp (p2A) coding for a RRM-containing protein was identified (Fig. 1A). This cDNA fragment was used to screen a T. cruzi Dm28c λEMBL3 genomic library (Fragoso and Goldenberg 1992) to obtain the corresponding full-lengh sequence and thereafter T. cruzi Dm28 clone (Contreras et al., 1988) was used. Two positive clones were obtained and sequenced both revealing an open reading frame of 471 bp coding for a 156 amino acid polypeptide with a predicted molecular weight of 17 kDa (Acc Number: EF451972). This protein that we initially named TcRBP17 has been named TcRBP19 (Acc Number: XP_814431) by De Gaudenzi et al. (De Gaudenzi et al., 2005) after the trypanosomatid genome database releases.
Fig 1.
Analysis of tcrbp19. (A) Comparison between the TcRBP19 RRM domain and the consensus RRM sequence obtained by BLAST (Altschul et al., 1990). The identities and similarities are boxed in black and gray respectively, gaps were introduced for best alignment. (B) Schematic representation of the protein domains in TcRBP19. The position of the RNP-1 and -2 motifs inside the RRM feature are indicated. Numbers indicate amino acid positions.
The comparison of the cDNA and the genomic sequence allowed the identification of the AG 5′ splice acceptor site 78 nucleotides upstream from the initial ATG. As usual, in trypanosomes this site is preceded by a polypyrimidine tract. The deduced isoelectric point of TcRBP19 is 6.96. A highly basic domain (pI 9.92) at the amino-terminal portion (aa from 1 to 30) and an acidic domain (pI 4.12) at the carboxy-terminal portion (aa from 115 to 156) that could be involved in protein-protein interactions are inferred (Fig. 1B). No relevant homologies were detected for these regions using the BLAST algorithm (Altschul et al., 1990). On the other hand, from the amino acid 31 to 114 there is a highly conserved RRM domain (PROSITE www.expasy.org) (Fig. 1B). The RRM domain is found in numerous proteins involved in post-transcriptional events such as RNA processing, transport, translation, degradation, and stabilization. It consists of 80–90 amino acid (aa) residues with two highly conserved short motifs: an RNP1 octamer, (K/R)G(F/Y)(G/A)FVX(F/Y), and an RNP2 hexamer, (L/I)(F/Y)(V/I)(G/K)(N/L)L (Kenan et al., 1991, Birney et al., 1993, Burd and Dreyfuss 1994,) also present in the TcRBP19 protein. TcRBP19 presents approximately 46% identical and 22% equivalent amino acids that are invariant in the RRM domain throughout the RRM protein family (BioEdit, ClustalW). These amino acids are involved in defining and stabilizing the active site of the protein. The most probably secondary structure for the entire protein was obtained by the PSIPRED Protein Structure Prediction Server (McGuffin et al., 2000). TcRBP19 may adopt a αβααββαββαβα conformation with the α2 being only 3 amino acid long, therefore the amino acids belonging to the RRM may form the expected βαββαβ topology (Varani and Nagai 1998, Perez-Canadillas and Varani 2001), where the RNP-1 and RNP-2 motives generate the β3 and β1 sheets respectively.
No remarkable homologies with the rest of the previously identified TcRBP RRM proteins from T. cruzi (Batista et al., 1994, Xu et al., 2001, De Gaudenzi et al., 2003, Gomes et al., 2004, De Gaudenzi et al., 2005) were found by BLASTp algorithm (Altschul et al., 1990). Instead, orthologues with high values of identity (from 46 to 59%) and similarity (from 60 to 70%) to TcRBP19 were found in different trypanosomatids: Trypanosoma brucei (Tb927.7.1180), Leishmania major (LmjF26.0760), Leishmania infantum (LinJ26.0600), Trypanosoma brucei gambiense (gamb1097f44.q1k_3), Trypanosoma vivax (tviv292c12.p1k_7).
The alignment of the RNP1 and RNP2 motifs from TcRBP19 using T-Coffee software (Notredame et al., 2000) with those from the above-mentioned trypanosomatid RRM orthologues evidences the high homologies at this level within the orthologous gene products (Table 1). Some peculiarities as the absence of an aromatic amino acid in position 3 and 2 for RNP1 and RNP2 respectively, can be observed. In other RRM proteins, these amino acids are responsible for non specific interactions with RNA (Oubridge et al., 1994; Allain et al., 1997; Price et al., 1998). The RNP-2 sub-motif is highly conserved in the RBP19 kinetoplastids orthologues, but it significantly differs from the consensus RNP2 sequence (Table 1). The significance of these peculiarities and their relatedness to the specific functional role awaits further analysis.
Table 1.
RNP-2 and RNP-1 alignments in TcRBP19 trypanosomatid orthologues.*
| RNP-2 | RNP-1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. cruzi | I | V | V | R | R | L | L | G | T | A | F | V | K | F |
| T. brucei (Tb927.7.1180) | I | V | I | R | N | I | L | G | T | A | F | V | K | F |
| T. brucei gambiense(gamb1097f44.q1k_3) | I | V | I | R | N | I | L | G | T | A | F | V | K | F |
| L. major (LmjF26.0760) | I | I | I | R | R | L | L | G | T | A | F | V | K | F |
| L. infantum (LinJ26.0600) | I | V | I | R | R | L | L | G | T | A | F | V | K | F |
| T. vivax (tviv292c12.p1k) | I | V | V | R | R | L | L | G | T | A | F | V | K | F |
|
| ||||||||||||||
| Consensus | L/I | F/Y | V/I | G/K | N/L | L | K/R | G | F/Y | G/A | F | V | X | F/Y |
identical amino acids between RNP-2 or RNP-1 and their corresponding consensus sequences in RRM proteins are shadowed
Southern blot analysis of T. cruzi genomic DNA digested with different endonucleases in high stringency conditions allowed us to conclude that tcrbp19 is a single copy gene (Fig. 2A). Two bands were observed when using Eco RI and Pst I since specific target sites for these enzymes are present within the tcrbp19 gene. Accordingly, BLAST analysis of the GeneDB Sanger Institute (http://www.genedb.org/) T. cruzi database enabled the detection of only two allelic sequences (Tc00.104.705.350.7515.60 and Tc00.104.705.350.8213.20) providing probability values P(N) of 8.7 and 2.6 e-79 respectively. Blot analysis of pulse field electrophoresis in high stringency conditions showed that tcrbp19 is located in a high molecular weight chromosome from T. cruzi (Fig. 2B). Using the databases of the genome projects (GeneDB) a second ORF coding for an RRM nearby tcrbp19 - distant ca. 2,000 pb- was detected. The presence of similarly clustered RRM proteins genes has been already observed in T. cruzi (De Gaudenzi et al., 2003).
Fig 2.
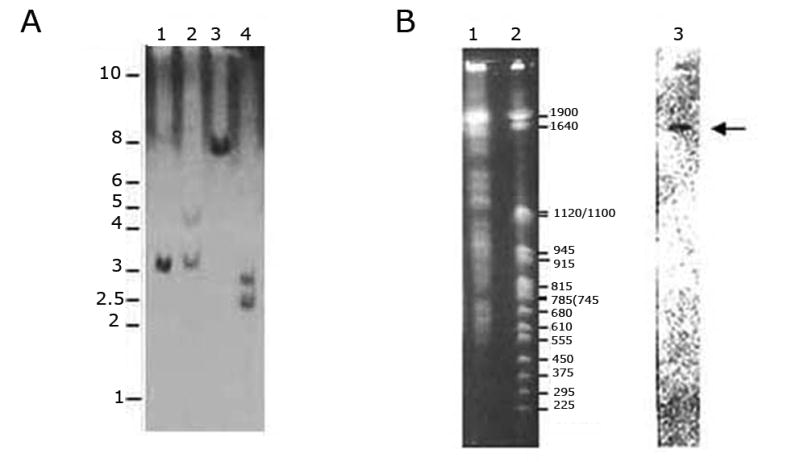
tcrbp19 is a single copy gene located in a high molecular weight chromosome. (A) Genomic analysis by Southern blot. 10 μg of genomic T. cruzi DNA prepared by phenol extraction and ethanol precipitation were digested with the restriction enzymes: lane 1: BamH I; lane 2: EcoR I; lane 3: Nco I; lane 4: Pst I, and subjected to a 0.7 % agarose gel electrophoresis. The transferred membrane was hybridized in high stringency conditions with a probe corresponding to p2A insert obtained by PCR in the presence of [α–32P]-dCTP. MW markers in kilobases (kb; 1kb ladder, PROMEGA) are indicated in the left of the panel. (B) Chromosomal localization. Total chromosomal DNA was prepared as reported (Garvey 1986) and separated by pulse field electrophoresis in a LKB 2015 Pusaphor System apparatus (Pharmacia LKB) as previously described (Dallagiovanna et al., 2001). The resulting gel was transferred and subjected to hybridization as indicated above: lane 1: T. cruzi chromosomal DNA (2 × 107 epimastigotes); lane 2: MW markers in kb (Yeast Chromosome PFG Marker, New England Biolabs); lane 3: autoradiography of lane 1 transferred membrane hybridized under high stringency conditions using the same probe as in A. Arrow points to the positive signal observed after hybridization.
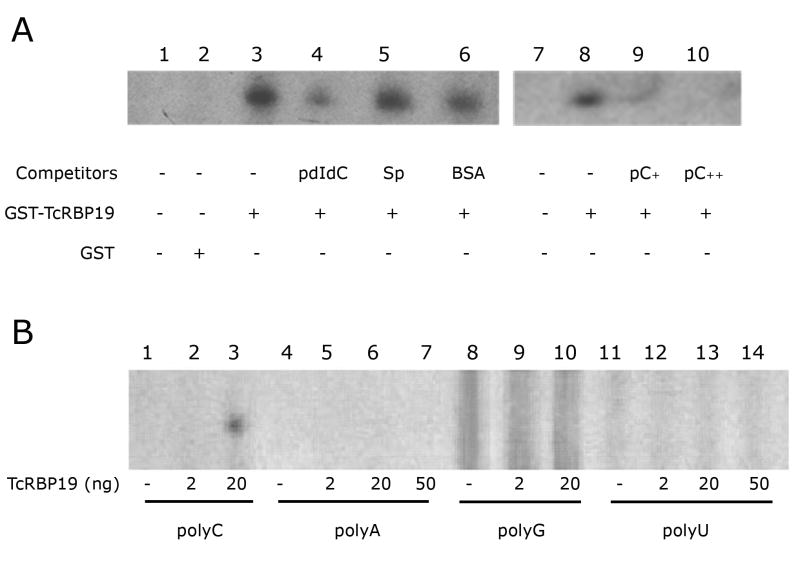
We then studied the ability of TcRBP19 to bind to homopolymeric ribonucleotides by electrophoretic mobility shift assay (EMSA). For this purpose we prepared the corresponding recombinant fusion protein as follows: two primers: BEfw 5′-TggatccCCGgaattcATGCATCAGCGAGGCATTCAGCG-3′ and NXrev 5′-GATgcggccgctcgagTCAGTGTGTCAATGTCTTTTCTCC-3′ were designed to obtain the complete TcRBP19 CDS by PCR amplification (lowercase indicates restriction sites). The PCR product containing BamHI-EcoRI and NotI-XhoI sites in the 5′ and 3′ ends respectively was cloned into the pCR®2.1-TOPO® plasmid (Invitrogen) and pGEX-4TI vector (GE Healthcare) and used to transform E. coli BL21 cells (Novagen). Cultures were induced with 0.5mM IPTG for 4 hr at 28°C to produce a glutathione-S-trasferase (GST) fusion protein (GST-TcRBP19). The recombinant protein, with a molecular weight of 45 kDa, was mainly obtained in the soluble fraction and purified using GST-SepharoseFF columns GSTrap FF (GE Healthcare) When tested by EMSA, the GST-TcRBP19 protein preferentially bound to the polyC sequence (Fig. 3A), but not to the polyU, polyA or polyG sequences (data not shown). The specificity of the GST-TcRBP19/polyC complex was further established by competition with non specific competitors (polydIdC, spermidine and BSA), unlabeled probes and also testing the binding capacity of the GST alone (Fig. 3 A). Afterwards, the recombinant fusion protein GST-TcRBP19 was digested with thrombin (GE Healthcare) at 16°C for 8 hrs to obtain the pure recombinant TcRBP19. This showed similar target specificity (Fig. 3B). Globally, these results indicate that TcRBP19 has a target discriminating potential. However, the actual targets remain unknown. Different approaches are currently being used in order to identify them.
Fig. 3.
TcRBP19 protein preferentially binds polyC in vitro. Binding reactions and electrophoretic mobility shift assays were performed as previously described (Duhagon et al., 2003). Oligoribonucleotides (15 nt long) were from IDT Co. Probes were end-labeled with T4 polynucleotide kinase (Roche) and [γ-32P]-ATP (GE Healthcare) following standard procedures (Ausubel 1987). (A) PolyC homoribonucleotide was radiolabeled with [γ-32P]-ATP and used as a probe for EMSA (labelled probe -LP-, 0,5 ng approx. 10,000 cpm per lane). Lane 1, free LP; lane 2, LP plus 1 μg GST; lane 3, LP plus 600 ng GST-TcRBP19; lanes 4 to 6, LP plus 800 ng GST-TcRBP and 1 μg polydIdC (lane 4), 10 mM spermidine (lane 5) or 1 μg BSA (lane 6). In lanes 9 and 10, the polyC ribonucleotide was added as an unlabeled competitor in 10- or 100-fold molar excess and incubated with 800 ng GST-TcRBP19 at room temperature for 10 minutes prior to the addition of the LP. (B) Polyhomoribonucleotides (polyC, polyA, polyG and polyU) were radiolabeled with [γ-32P]-ATP and used as a probe for EMSA (0.5 ng approx. 10,000 cpm per lane), alone or in combination with TcRBP19 recombinant protein (2, 20 or 50 ng). The gels were pre-run at 200 volts at 4°C and then electrophoresed with the samples for 3 to 4 hours at 250 volts at 4 °C. Next, they were dried and used to expose X-ray films at −80 °C.
In order to study TcRBP19 expression during the parasite life cycle, a polyclonal antiserum was raised in New Zealand White rabbits by immunization with the recombinant fusion protein GST-TcRBP19 using Freund's adjuvant. Rabbits were inoculated sub-cutaneously three times, at two-week intervals, with the protein (200 μg each time) and serum was obtained two weeks after the last boost. The polyclonal serum was purified on DEAE Affi-Gel®Blue Gel columns (BioRad) following manufacturer's instructions. Afterwards affinity purification using GST-TcRBP19 bound to Affi-Gel 10 Gel columns (BioRad) and GST-TcRBP19 bound to Hybond-C was performed. The purified TcRBP19 antibody was used in Western blot to analyze protein extracts of E. coli BL21 transformed with the pGEX-4TI-TcRBP19 expression plasmid. Antibody dilutions of 1/1000 were able to specifically recognize the TcRBP19 protein (Fig. 4A). Nevertheless, when used in Western blot to analyze epimastigote protein extracts a unique band of an unexpected slow migration corresponding to an apparent molecular mass of 45 kDa was detected (Fig. 4A). This was not due to dimer formation because treatments with 1 and 2M DTT or 5% β–mercaptoethanol showed the same pattern (data not shown). We then used the antibody to analyze different parasite stages prepared as previously reported (Contreras et al., 1985, 2002). The sole band of 45 kDa was also detected in metacyclic trypomastigotes, but hardly in amastigotes (Fig. 4A). Since a posttranslational modification could be responsible for the slow migration signal, we decided to prepare transfectants of T. cruzi over expressing the TcRBP19 protein. The low expression plasmid pTEX (Kelly et al., 1992) was chosen to transfect epimastigotes of T. cruzi. The TcRBP19 gene was inserted in the BamH I and Xho I sites to construct the plasmid pTEX-TcRBP19. Dm28 T. cruzi epimastigotes (5×107) were electroporated using 50 μg of pTEX-TcRP19 with two pulses of 450 V 500 μF for two replicates (A and B). Electroporations with the plasmid pTEX alone and with no plasmid were done as negative controls. Electroporated parasites were incubated in LIT medium supplemented with 10 % FBS for 24 hours. Resistance to genetecin using 250 μg/mL for the first 48 hours and then 500 μg/mL, achieved selection of stable transfectants. Cultures were followed by optical microscopy. Twelve days later, live parasites were observed only in the cultures corresponding to transfectants pTEX and pTEX-TcRBP19. The over expression of the TcRBP19 protein introduces neither evident morphological changes at optical microscopy observation nor alterations on the growth curve when compared with the control pTEX T. cruzi transfectants (data not shown). Transfectants were checked by PCR with vector primers. Interestingly, Western blots of total proteins from the transfectants using the purified anti-TcRBP19 antibody showed a clear over expression of a product of 17 kDa meanwhile no evident increase in the content of the 45 kDa signal was observed (Fig. 4B). We then hypothesized that TcRBP19 could be a very low expression protein and decided to use [125I] protein A in the Western blot to amplify the signal. A faint signal migrating at 17 kDa was observed in the amastigotes (Fig. 4C). In order to normalize the protein level loaded into the well the constitutive expression of phospho-enol-pyruvate carboxykinase (PEPCK) was checked (Fig. 4D). This approach allowed as to conclude that the expression of TcRBP19 is only barely and mainly restricted to the amastigote form. It is of note that Northern blots using epimastigote total RNA were not able to detect the presence of the tcrbp19 mRNA (data not shown). Moreover, we were unable to amplify the 3′UTR of the gene by RT-PCR from epimastigote polyA mRNA. Accordingly, the sequence of tcrbp19 is not present in the EST database of the T. cruzi genome project. These findings suggest a strict control of tcrbp19 transcript abundance.
Fig. 4.
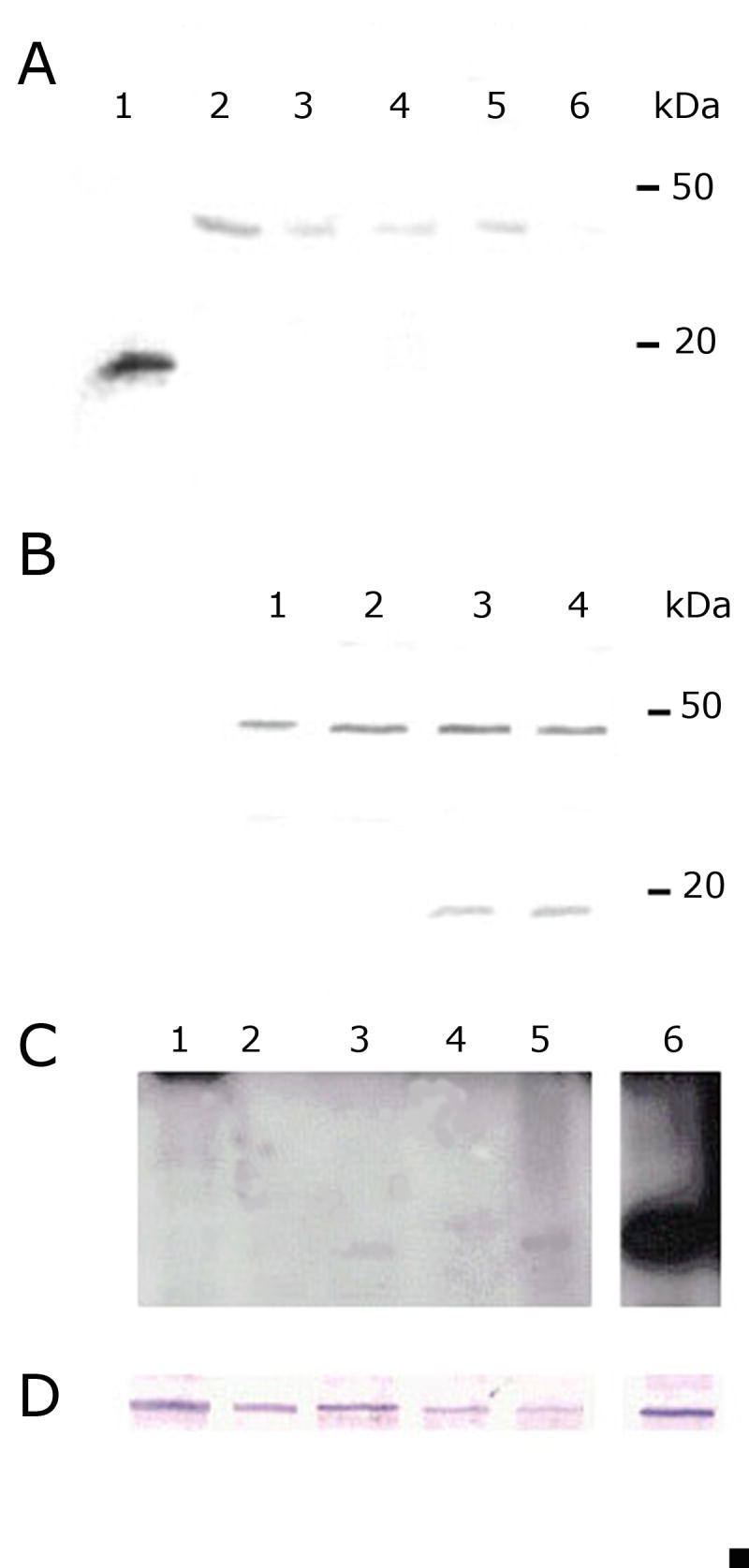
TcRBP19 protein is a low level differentially expressed protein. Protein extracts were separated by electrophoresis in 15% SDS-polyacrylamide gels and electro-transferred onto Hybond-C membranes (GE Healthcare) following standard procedures. Blots were blocked by incubation in 5 % skim milk powder in PBS and where then incubated with the purified polyclonal anti-TcRBP19 antibody in PBS-Tween buffer for 1 hour at room temperature. Bound antibodies were detected using peroxidase conjugated AffiniPure goat anti-rabbit IgG (H+L) (Jackson ImmunoResearch) diluted 1/7,500, with the color reaction developed using 5 mg of DAB (Sigma) in 10 mL 0.05 M Tris pH 7.6 added with 10 μL 30% H2O2. Alternatively, after extensive washings in PBS-Tween-5 % skim milk, the filter was incubated with [125I] labeled protein-A (approx 5.105 cpm/mL) (GE Healthcare), extensively washed and exposed to X-ray film (Hyperfilm, GE Healthcare, UK). (A) Life cycle of wild type parasites. Total protein extracts corresponding to 5×106 parasites per lane. Lane 1: 200ng of purified TcRBP19 recombinant protein; lane 2: epimastigotes 3 days; lane 3: epimastigotes 5 days; lane 4: 24 hours of TAU3AAG nutritional stressed epimatigotes; lane 5: metacyclic trypomastigotes; lane 6: amastigotes Molecular weights are indicated. (B) TcRBP19 over expressing epimastigotes. Total protein extracts corresponding to 107 parasites per lane. Lane 1: epimastigotes wt; lane 2: epimastigotes transfected with pTEX; lane 3: epimastigotes transfected with pTEX-TcRBP19; lane 4: epimastigotes transfected in replica with pTEX-TcRBP19. Molecular weights are indicated. (C) Western blot using [125I] labeled protein-A. Total protein extracts corresponding to 108 parasites per lane. Lane 1: epimastigotes; lane 2: metacyclic trypomastigotes; lane 3: axenic amastigotes; lane 4: trypomastigotes; lane 5: cellular amastigotes; lane 6. epimastigotes transfected with pTEX-TcRBP19. (D) Western blot using the same extracts of panel C using anti-PEPCK antibody as internal control.
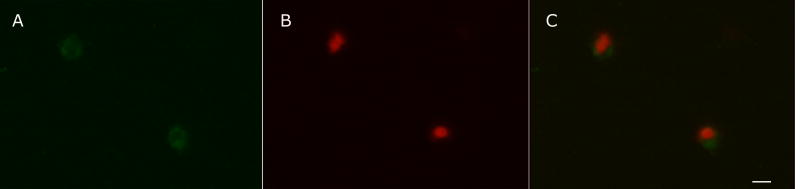
Due to the low level of TcRBP19 expression in wild type T. cruzi, subcellular localization was studied using the over expressing parasites. Confocal immunofluorescence analysis of in vitro differentiated amastigotes showed a diffuse cytoplasmic reactivity with the specific anti-TcRBP19 antibody obtained by us. No signal was evident in the nuclei (Fig. 5).
Fig. 5.
TcRBP19 is located in the T. cruzi amastigote cytoplasm. Localization was performed as described previously (Dallagiovanna et al., 2005). Parasites were harvested from culture media, washed four times with PBS and resuspended (5 × 106 cells/mL). Fixed cells (4% paraformaldehyde in PBS) were blocked with PBS-Tween, BSA 1% for 30 min., incubated with anti-GST-TcRBP19 for one hour and then with antirabbit Alexa-488 F(ab') fragment of goat antirabbit IgG (H+L) (Molecular probes). Counterstaining was done using propidium iodide. Images were obtained using a FV300 Olympus Confocal microscope using a 100x lens (oil, N.A.1.35) with Argon (488nm) and HeNe (546nm) laser excitation. (A) Single (∼0.5μm thick) confocal section showing that TcRBP19 (green) localizes in the small cytoplasmic ring of over expressing amastigotes. (B) Nuclei stained with propidium iodide (red). (C) Image A and B were superimposed. Scale bar is 5μm.
In conclusion, TcRBP19 is a cytoplasmic low expression T. cruzi protein only barely detected in the amastigote stage. It is coded by a single copy gene located in a high molecular weight chromosome. TcRBP19 is an RNA binding protein that presents discriminating ability among the different homoribonucleotides. It contains an RRM motif with a peculiar RNP2 composition highly conserved among the orthologous products but showing only a limited resemblance with the consensus motif. No insight into the function of TcRBP19 could be achieved by a sequence homology search. However, the above mentioned characteristics suggest the involvement of TcRBP19 in specific or early regulatory steps in T. cruzi. The RRM represents a common motif present in a variety of eukaryotic proteins that bind different RNA targets such as hnRNA, snRNA, polyA and polyPy among others (Burd and Dreyfuss, 1994). The roles of the majority of the RRM proteins in kinetoplastids are still not determined. Indeed, by homology, a function could be assigned with confidence to less than 25% of the 75 RRM proteins in T. brucei (De Gaudenzi et al., 2005). In this context the data here provided constitute a first step to the characterization of one such protein. Work is in progress to achieve the functional role of TcRBP19.
Acknowledgments
This work was financially supported by FIRCA n° R03 TW05665-01, Fondo Clemente Estable (DICyT) n° 7109 and n° 169, and CNPq Prosul program. The authors are grateful for their fellowships: LP, MAD and PS (PEDECIBA), BD (FIOCRUZ-CNPq), SG and MAK (CNPq), BG (PV-CNPq), LP and PS (RTPD-SIDA) and MAD (AMSUD-Pasteur).
Index descriptors and Abbreviations
- GST
Glutathione-S-transferase
- RBP
RNA binding proteins
- RRM
RNA recognition motif
- SL
spliced leader
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Allain FHT, Howe PWA, Neuhaus D, Varani G. Structural basis of the RNA-binding specificity of human U1A protein. The EMBO Journal. 1997;16:5764–5774. doi: 10.1093/emboj/16.18.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM. Current protocols in molecular biology. Greene Pub. Associates and Wiley-Interscience: J. Wiley; New York: 1987. [Google Scholar]
- Batista JA, Teixeira SM, Donelson JE, Kirchhoff LV, de Sa CM. Characterization of a Trypanosoma cruzi poly(A)-binding protein and its genes. Molecular and Biochemical Parasitology. 1994;67:301–312. doi: 10.1016/0166-6851(94)00133-2. [DOI] [PubMed] [Google Scholar]
- Birney E, Kumar S, Krainer AR. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Research. 1993;21:5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- Caro F, Bercovich N, Atorrasagasti C, Levin MJ, Vazquez MP. Protein interactions within the TcZFP zinc finger family members of Trypanosoma cruzi: implications for their functions. Biochemical and Biophysical Research Communication. 2005;333:1017–1025. doi: 10.1016/j.bbrc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Caro F, Bercovich N, Atorrasagasti C, Levin MJ, Vazquez MP. Trypanosoma cruzi: Analysis of the complete PUF RNA-binding protein family. Experimental Parasitology. 2006;113:112–124. doi: 10.1016/j.exppara.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Contreras VT, Araujo-Jorge TC, Bonaldo MC, Thomaz N, Barbosa HS, Meirelles Mde N, Goldenberg S. Biological aspects of the Dm 28c clone of Trypanosoma cruzi after metacyclogenesis in chemically defined media. Memorias do Instituto Oswaldo Cruz. 1988;83:123–133. doi: 10.1590/s0074-02761988000100016. [DOI] [PubMed] [Google Scholar]
- Contreras VT, Navarro MC, De Lima AR, Arteaga R, Duran F, Askue J, Franco Y. Production of amastigotes from metacyclic trypomastigotes of Trypanosoma cruzi. Memorias do Instituto Oswaldo Cruz. 2002;97:1213–1220. doi: 10.1590/s0074-02762002000800025. [DOI] [PubMed] [Google Scholar]
- Contreras VT, Salles JM, Thomas N, Morel CM, Goldenberg S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Molecular and Biochemical Parasitology. 1985;16:315–327. doi: 10.1016/0166-6851(85)90073-8. [DOI] [PubMed] [Google Scholar]
- D'Orso I, De Gaudenzi JG, Frasch AC. RNA-binding proteins and mRNA turnover in trypanosomes. Trends in Parasitology. 2003;19:151–155. doi: 10.1016/s1471-4922(03)00035-7. [DOI] [PubMed] [Google Scholar]
- D'Orso I, Frasch AC. TcUBP-1, a developmentally regulated U-rich RNA-binding protein involved in selective mRNA destabilization in trypanosomes. Journal of Biological Chemistry. 2001;276:34801–34809. doi: 10.1074/jbc.M102120200. [DOI] [PubMed] [Google Scholar]
- D'Orso I, Frasch AC. TcUBP-1, an mRNA destabilizing factor from trypanosomes, homodimerizes and interacts with novel AU-rich element- and Poly(A)-binding proteins forming a ribonucleoprotein complex. Journal of Biological Chemistry. 2002;277:50520–50528. doi: 10.1074/jbc.M209092200. [DOI] [PubMed] [Google Scholar]
- Dallagiovanna B, Perez L, Sotelo-Silveira J, Smircich P, Duhagon MA, Garat B. Trypanosoma cruzi: Molecular characterization of TcPUF6, a Pumilio protein. Experimental Parasitology. 2005;109:260–264. doi: 10.1016/j.exppara.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Dallagiovanna B, Plazanet-Menut C, Ogatta SF, Avila AR, Krieger MA, Goldenberg S. Trypanosoma cruzi: a gene family encoding chitin-binding-like proteins is posttranscriptionally regulated during metacyclogenesis. Experimental Parasitology. 2001;99:7–16. doi: 10.1006/expr.2001.4628. [DOI] [PubMed] [Google Scholar]
- De Gaudenzi J, Frasch AC, Clayton C. RNA-Binding Domain Proteins in Kinetoplastids: a Comparative Analysis. Eukaryot Cell. 2005;4:2106–2114. doi: 10.1128/EC.4.12.2106-2114.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gaudenzi JG, D'Orso I, Frasch AC. RNA recognition motif-type RNA-binding proteins in Trypanosoma cruzi form a family involved in the interaction with specific transcripts in vivo. Journal of Biological Chemistry. 2003;278:18884–18894. doi: 10.1074/jbc.M301756200. [DOI] [PubMed] [Google Scholar]
- Duhagon MA, Dallagiovanna B, Ciganda M, Ruyechan W, Williams N, Garat B. A novel type of single-stranded nucleic acid binding protein recognizing a highly frequent motif in the intergenic regions of Trypanosoma cruzi. Biochemical and Biophysical Research Communication. 2003;309:183–188. doi: 10.1016/s0006-291x(03)01561-4. [DOI] [PubMed] [Google Scholar]
- El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, Tran AN, Ghedin E, Worthey EA, Delcher AL, Blandin G, Westenberger SJ, Caler E, Cerqueira GC, Branche C, Haas B, Anupama A, Arner E, Aslund L, Attipoe P, Bontempi E, Bringaud F, Burton P, Cadag E, Campbell DA, Carrington M, Crabtree J, Darban H, da Silveira JF, de Jong P, Edwards K, Englund PT, Fazelina G, Feldblyum T, Ferella M, Frasch AC, Gull K, Horn D, Hou L, Huang Y, Kindlund E, Klingbeil M, Kluge S, Koo H, Lacerda D, Levin MJ, Lorenzi H, Louie T, Machado CR, McCulloch R, McKenna A, Mizuno Y, Mottram JC, Nelson S, Ochaya S, Osoegawa K, Pai G, Parsons M, Pentony M, Pettersson U, Pop M, Ramirez JL, Rinta J, Robertson L, Salzberg SL, Sanchez DO, Seyler A, Sharma R, Shetty J, Simpson AJ, Sisk E, Tammi MT, Tarleton R, Teixeira S, Van Aken S, Vogt C, Ward PN, Wickstead B, Wortman J, White O, Fraser CM, Stuart KD, Andersson B. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science. 2005;309:409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- Espinosa JM, Portal D, Lobo GS, Pereira CA, Alonso GD, Gomez EB, Lan GH, Pomar RV, Flawia MM, Torres HN. Trypanosoma cruzi poly-zinc finger protein: a novel DNA/RNA-binding CCHC-zinc finger protein. Molecular and Biochemical Parasitology. 2003;131:35–44. doi: 10.1016/s0166-6851(03)00187-7. [DOI] [PubMed] [Google Scholar]
- Fragoso SP, Goldenberg S. Cloning and characterization of the gene encoding Trypanosoma cruzi DNA topoisomerase II. Molecular and Biochemical Parasitology. 1992;55:127–134. doi: 10.1016/0166-6851(92)90133-5. [DOI] [PubMed] [Google Scholar]
- Garvey E, Santi DV. Stable amplified DNA in drug-resistant Leishmania exists as extrachromosomal circles. Science. 1986;233:535–540. doi: 10.1126/science.3726545. [DOI] [PubMed] [Google Scholar]
- Gomes GG, Peter Urmenyi T, Rondinelli E, Williams N, Silva R. TcRRMs and Tcp28 genes are intercalated and differentially expressed in Trypanosoma cruzi life cycle. Biochemical and Biophysical Research Communication. 2004;322:985–992. doi: 10.1016/j.bbrc.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, Sisk E, Rajandream MA, Adlem E, Aert R, Anupama A, Apostolou Z, Attipoe P, Bason N, Bauser C, Beck A, Beverley SM, Bianchettin G, Borzym K, Bothe G, Bruschi CV, Collins M, Cadag E, Ciarloni L, Clayton C, Coulson RM, Cronin A, Cruz AK, Davies RM, De Gaudenzi J, Dobson DE, Duesterhoeft A, Fazelina G, Fosker N, Frasch AC, Fraser A, Fuchs M, Gabel C, Goble A, Goffeau A, Harris D, Hertz-Fowler C, Hilbert H, Horn D, Huang Y, Klages S, Knights A, Kube M, Larke N, Litvin L, Lord A, Louie T, Marra M, Masuy D, Matthews K, Michaeli S, Mottram JC, Muller-Auer S, Munden H, Nelson S, Norbertczak H, Oliver K, O'Neil S, Pentony M, Pohl TM, Price C, Purnelle B, Quail MA, Rabbinowitsch E, Reinhardt R, Rieger M, Rinta J, Robben J, Robertson L, Ruiz JC, Rutter S, Saunders D, Schafer M, Schein J, Schwartz DC, Seeger K, Seyler A, Sharp S, Shin H, Sivam D, Squares R, Squares S, Tosato V, Vogt C, Volckaert G, Wambutt R, Warren T, Wedler H, Woodward J, Zhou S, Zimmermann W, Smith DF, Blackwell JM, Stuart KD, Barrell B, Myler PJ. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JM, Ward HM, Miles MA, Kendall G. A shuttle vector which facilitates the expression of transfected genes in Trypanosoma cruzi and Leishmania. Nucleic Acids Research. 1992;20:3963–3969. doi: 10.1093/nar/20.15.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenan DJ, Query CC, Keene JD. RNA recognition: towards identifying determinants of specificity. Trends in Biochemical Sciences. 1991;16:214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- LeBowitz JH, Smith HQ, Rusche L, Beverley SM. Coupling of poly(A) site selection and trans-splicing in Leishmania. Genes & Development. 1993;7:996–1007. doi: 10.1101/gad.7.6.996. [DOI] [PubMed] [Google Scholar]
- Manning-Cela R, Gonzalez A, Swindle J. Alternative splicing of LYT1 transcripts in Trypanosoma cruzi. Infection and Immunity. 2002;70:4726–4728. doi: 10.1128/IAI.70.8.4726-4728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- Morking PA, Dallagiovanna BM, Foti L, Garat B, Picchi GF, Umaki AC, Probst CM, Krieger MA, Goldenberg S, Fragoso SP. TcZFP1: a CCCH zinc finger protein of Trypanosoma cruzi that binds poly-C oligoribonucleotides in vitro. Biochemical and Biophysical Research Communication. 2004;319:169–177. doi: 10.1016/j.bbrc.2004.04.162. [DOI] [PubMed] [Google Scholar]
- Oubridge C, Ito N, Evans PR, Teo CH, Nagai K. Crystal structure at 1.92 A resolution of the RNA-binding domain of the U1A spliceosomal protein complexed with an RNA hairpin. Nature. 1994;372:432–438. doi: 10.1038/372432a0. [DOI] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302(1):205–17. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- Perez-Canadillas JM, Varani G. Recent advances in RNA-protein recognition. Current Opinion in Structural Biology. 2001;11:53–58. doi: 10.1016/s0959-440x(00)00164-0. [DOI] [PubMed] [Google Scholar]
- Price SR, Evans PR, Nagai K. Crystal structure of the spliceosomal U2B″-U2A′ protein complex bound to a fragment of U2 small nuclear RNA. Nature. 1998;394:645–650. doi: 10.1038/29234. [DOI] [PubMed] [Google Scholar]
- Robello C, Navarro P, Castanys S, Gamarro F. A pteridine reductase gene ptr1 contiguous to a P-glycoprotein confers resistance to antifolates in Trypanosoma cruzi. Molecular and Biochemical Parasitology. 1997;90:525–535. doi: 10.1016/s0166-6851(97)00207-7. [DOI] [PubMed] [Google Scholar]
- Teixeira SM. Control of gene expression in Trypanosomatidae. Brazilian Journal of Medical and Biological Research. 1998;31:1503–1516. doi: 10.1590/s0100-879x1998001200001. [DOI] [PubMed] [Google Scholar]
- Varani G, Nagai K. RNA recognition by RNP proteins during RNA processing. Annual Review of Biophysics & Biomolecular Structure. 1998;27:407–445. doi: 10.1146/annurev.biophys.27.1.407. [DOI] [PubMed] [Google Scholar]
- Xu P, Wen L, Benegal G, Wang X, Buck GA. Identification of a spliced leader RNA binding protein from Trypanosoma cruzi. Molecular and Biochemical Parasitology. 2001;112:39–49. doi: 10.1016/s0166-6851(00)00341-8. [DOI] [PubMed] [Google Scholar]