Abstract
We have previously established an in vivo requirement for GATA4 and FOG2 transcription factors in sexual differentiation. Fog2 null mouse fetuses or fetuses homozygous for a targeted mutation in Gata4 (Gata4ki), which cripples the GATA4-FOG2 interaction, exhibit a profound and early block in testis differentiation in both sexes. Others have shown that XX mice with the Ods transgenic insertion or the Wt1-Sox9 YAC transgene overexpress the testis differentiation gene, Sox9. Thus, these XX animals undergo dominant sex-reversal by developing into phenotypically normal, but sterile, males. Now we have determined that Fog2 haploinsufficiency prevents (suppresses) this dominant sex-reversal and Fog2+/− Wt1-Sox9 or Ods XX animals develop normally - as fertile females. The suppression of sex-reversal in Fog2 heterozygous females results from approximately 50% downregulation of the expression from the transgene-associated allele of Sox9. The GATA4/FOG2-dependent sex reversal observed in the transgenic XX gonads has to rely on gene targets other than the Y chromosome-linked Sry gene. Importantly, Fog2 null or Gata4ki/ki embryos (either XX or XY) fail to express detectable levels of Sox9 despite carrying the Ods mutation or Wt1-Sox9 transgene. Fog2 haploinsufficiency leads to a decreased amount of SOX9-positive cells in XY gonads. We conclude that FOG2 is a limiting factor in the formation of a functional GATA4/FOG2 transcription complex that is required for Sox9 expression during gonadogenesis.
INTRODUCTION
The basic principle of mammalian sexual determination is that genetic sex is already determined by the presence of the Y chromosome at fertilization. However, male and female embryos are morphologically indistinguishable during their early development; in both sexes the bipotential (indifferent) gonads arise from the urogenital ridges that appear on the surface of mesonephroi, a bilateral rudimentary nephric organ that lies parallel to the differentiating gonad. At a specific developmental stage the male and the female pathways diverge: the XY gonadal anlagen differentiate into testes and the XX anlagen form ovaries (Capel, 2000). This sex determination step in mammals is initiated by Sry, the Y chromosome-linked testis-determining gene. Triggered by SRY, formation of the testes rather than ovaries from a bipotential embryonic gonad is the decisive step for subsequent male sexual development. The molecular events that set Sry in motion and culminate in testis formation remain to be defined.
One of the major downstream targets of SRY in testis is thought to be Sox9. Sox9 expression appears to be both necessary and sufficient for testis development; in mice, Sox9 alone is sufficient to initiate testis differentiation, independent of Sry (Bishop et al., 1999; Qin and Bishop, 2005; Vidal et al., 2001). However, Sox9 expression is not induced in the absence of Sry; thus one of the functions of Sry is to activate Sox9 gene expression. Although the genetic relationship between Sry and Sox9 has been established for quite some time, the mechanism of Sox9 activation by SRY still remains enigmatic. In addition to a direct activation model of SRY acting through the Sox9 cis-elements (reviewed in (Kanai et al., 2005; Koopman, 1999), it has been also hypothesized that SRY interferes either with the synthesis of a repressor of Sox9 (yet unidentified) (McElreavey et al., 1993) or with the binding of this putative repressor to a Sox9 enhancer (Bishop et al. 1999).
An interest in understanding the transcriptional regulation of Sox9 expression has been also driven by the involvement of SOX9 mutation in human disease as heterozygous defects in SOX9 are associated with the skeletal malformation syndrome (campomelic dysplasia, CD) in humans. The observation that a large proportion of CD patients also experience XY sex reversal revealed a role for SOX9 in human sexual development. Importantly, in some patients chromosome rearrangements were found from 50 kb to 950 kb upstream of SOX9 (Foster et al., 1994; Pfeifer et al., 1999; Wagner et al., 1994) thus implicating a long-range control for this gene. The involvement of a remote control element gained further support by the fact that mice transgenic for human SOX9-spanning YACs showed transgene expression patterns similar to those in endogenous Sox9, but only when the YAC transgene contained a 350-kb sequence upstream of SOX9 (and approximately 250 kb of the 3’-flanking sequence) and not with a truncated YAC that contained only 75 kb of a 5’flanking sequence (Wunderle et al., 1998). Importantly even with these substantial (5’-350kb and 3’-250kb) flanking regions, gonadal expression from the YAC was not observed, thus leading one to propose that the SOX9/Sox9 gonadal elements could reside even further upstream/downstream (Wunderle et al., 1998). These data have to be reconciled, however, with the reported observation that approximately 70 kb of the 5’- and 30 kb of the 3’-flanking sequence were sufficient for the testis-specific expression of Sox9 (Lovell-Badge et al., 2002).
We have previously shown an in vivo requirement for GATA4 and its co-factor FOG (Friend of GATA)-2 transcription factors in testis differentiation (Tevosian et al., 2002). Fog2 null (Tevosian et al., 2000) and Gata4ki/ki mutant (Crispino et al., 2001) XY gonads are able to initiate the expression of Sry (albeit at the substantially lower level compared to the wild-type controls), but not of Sox9 (Tevosian et al., 2002). Hence, GATA4 /FOG2 function could be required for Sox9 activation. Given the pivotal position of Sox9 in gonad differentiation, we hypothesized that the absence of Sox9 expression could be sufficient to cause the early and severe block in the development of Gata4ki/ki and Fog2 null mutant testis.
It remained unclear, however, whether GATA4/FOG2 complex plays an essential (or any at all) role in testis differentiation subsequent to Sry activation. As mutations in GATA4/FOG2 lead to a significant decrease in the expression of the Sry gene (Tevosian et al., 2002), it was possible that concomitant loss of its primary target - Sox9 expression - is indirect and results solely from the down-regulation of Sry. We sought to examine this possibility using the Odd sex (ocular degeneration with sex reversal, Ods) line of animals. In these mice, a fortuitous insertion of the tyrosinase transgene results in mis-regulation (a high level of expression) of the Sox9 gene in the XX gonad; this high expression of Sox9 in XX mice results in a dominant, female-to-male, sex-reversal (Bishop et al., 1999). Since Ods allele of Sox9 still retains all of the elements necessary to specifically activate the Sox9 gene in the supporting cells of the gonad (Qin et al., 2003) we could derive the information about Sox9 regulation by GATA4/FOG2. Importantly, the ability of the GATA4/FOG2 complex to regulate the Sox9 (Ods) in the XX gonads (in the absence of the Y chromosome) has to be independent from its ability to regulate the Sry, the Y chromosome-linked gene.
To further determine whether the loss of Fog2 affects the steps in the testis differentiation program subsequent to Sox9 induction, we used another line of transgenic mice, Wt1-Sox9. In these animals Sox9 is expressed from the Wt-1 regulatory elements within a yeast artificial chromosome (YAC), faithfully mimicking gonadal expression of the endogenous Wt1 gene (Vidal et al., 2001). Wt1 is expressed normally in the Fog2 null gonads (Tevosian et al., 2002) and we anticipated that crossing the Wt1-Sox9 animals into a Fog2−/− background should result in Fog2-independent Sox9 expression. Hence, in the XY and XX Wt1-Sox9 transgenic embryos one could potentially separate the SOX9-dependence and GATA4/FOG2-dependence for genes in the testis differentiation program. For example, it has been shown that the Müllerian inhibitory substance (Mis) gene is an in vivo target of SOX9 (Arango et al., 1999); there is also evidence that GATA sites (and GATA4 protein) are essential in the Mis/MIS promoter regulation (Viger et al., 1998; Watanabe et al., 2000). In the Fog2 null or GATA4ki/ki XY embryos neither Sox9 nor Mis are expressed; by restoring Sox9 expression with Wt1-Sox9 in Gata4 or Fog2 mutants we could determine whether SOX9 is still able to activate Mis in the absence of the functional GATA4/FOG2 complex.
Unexpectedly, we have now determined that Fog2 haploinsufficiency prevents (suppresses) sex-reversal and Fog2+/− Wt1-Sox9 or Ods XX animals develop normally - as fertile females. The suppression of sex-reversal in Fog2 heterozygous females results from an approximately 50% downregulation of the expression from the transgene-associated allele of Sox9. The GATA4/FOG2-dependent sex reversal observed in the transgenic XX gonads has to rely on gene targets other than the Y chromosome-linked Sry gene. Importantly, Fog2 null or Gata4ki/ki embryos (either XX or XY) fail to express detectable levels of Sox9 despite carrying the Ods mutation or Wt1-Sox9 transgene. We also show that Fog2 haploinsufficiency results in a decreased amount of SOX9-positive cells in XY gonads. We conclude that FOG2 is a limiting factor in the formation of a functional GATA4/FOG2 transcription complex that is required for Sox9 expression during gonadogenesis.
RESULTS
Induction of dominant XX sex reversal requires two functional Fog2 alleles
Fog2 deletion is embryonic lethal in mice (Tevosian et al., 2000). To generate the Ods/+ or Wt1-Sox9/+ Fog2−/− embryos it was thus necessary to initially introduce these alleles into Fog2+/− background and then backcross the resulting Wt1-Sox9/+ Fog2+/− or Ods/+ Fog2+/− males with the Fog2+/− females. The Ods mutation, in addition to causing a female-to-male sex reversal, also results in the characteristic eye phenotype of micropthalmia with cataracts (Bishop et al., 1999; Qin et al., 2004). To our surprise, among the progeny of the first cross (XY Ods/+ x XX Fog2+/−), we observed several phenotypic females with the eye phenotype. PCR genotyping of these females confirmed the presence of the Ods mutation (3 out of 3); however, in determining their Fog2 status we also noticed that all of these animals are heterozygous for the Fog2 mutation (Fig. 1A). In the Wt1-Sox9 cross (XY Wt1-Sox9/+ x XX Fog2+/−) all of the F1 phenotypic female transgenics (XX Wt1-Sox9) were similarly positive for the Fog2 null allele (5 out of 5). In summary, 100% (53/53) of the XX Ods/+Fog2+/− and 100% (62/62) of the XX Wt1-Sox9/+ Fog2+/− animals remained phenotypic females; all tested females were fertile (delivered offspring). To confirm the absence of sex reversal in these Fog2+/− females we have performed a qRT-PCR for follistatin and Bmp2 RNA in XX E12.5 gonads. We found no significant difference between these markers in the control (Bmp2: 100%, Fst: 100%), OdsFog2+/− (Bmp2: 93 ± 12%; Fst: 94±15%) and Wt1-Sox9Fog2+/− (Bmp2 108±12%; Fst:110±15%) females. We also isolated ovaries from the adult XX Ods Fog2+/− mice and found them grossly normal upon histological examination (Supplemental Figure 1).
Figure 1.
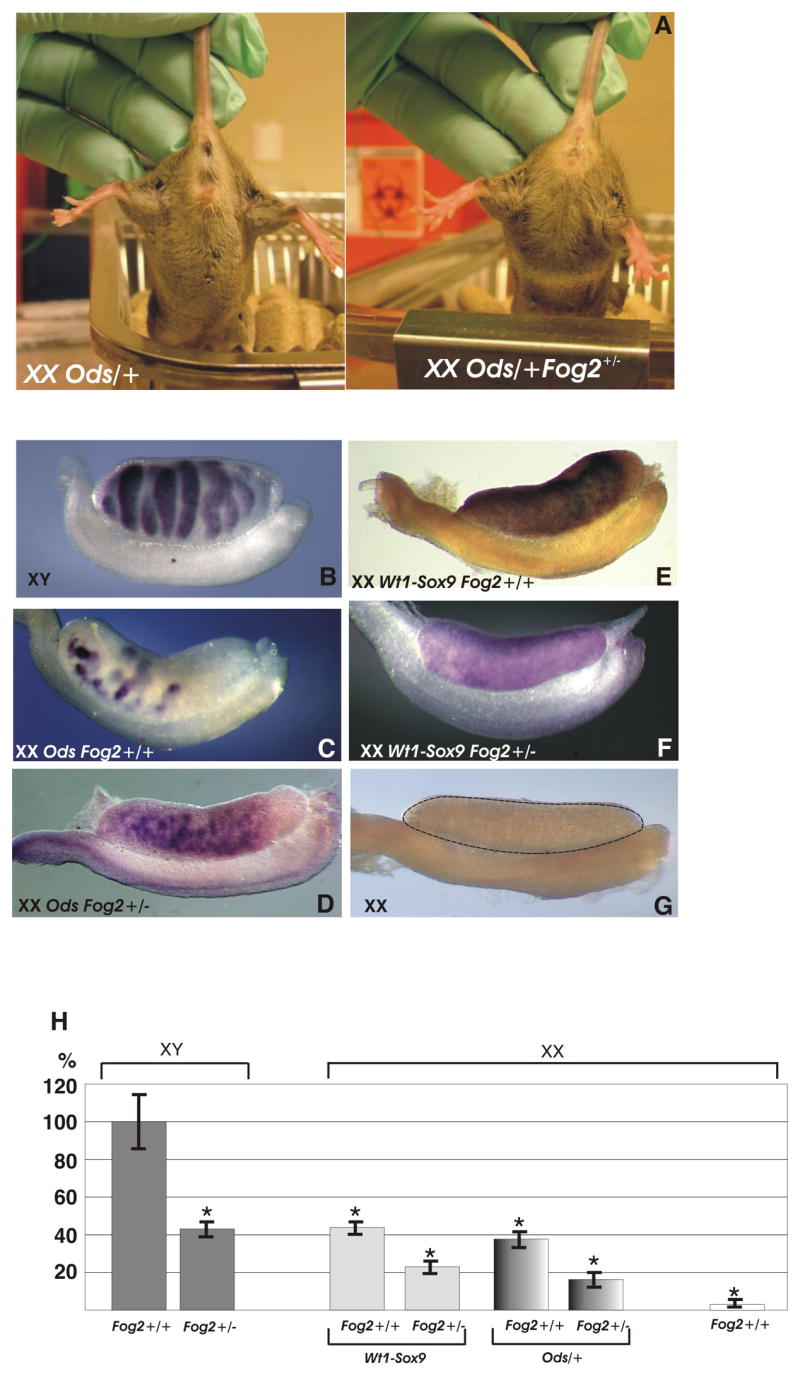
(A) An XX Ods/+ Fog2+/+ and an XX Ods/+ Fog2+/− transgenic littermates are shown. The Ods/+ mouse has male external genitalia (left), while the OdsFog2+/− heterozygous animal is a fertile female (right). (B-) Whole mount in situ hybridization analysis of the Sox9 expression in the E13.5 embryonic male (B), XX sex-reversed (C and E) and XX Fog2 heterozygous (D and F) gonads. Sox9 expression is present in all the samples with the exception of the E13.5 female (G). (F) Real-time PCR analysis (TaqMan) of Sox9 expression in the E12.5 embryonic gonads.
We concluded that the induction of sex reversal in these two models requires a full complement of the functional Fog2 gene.
XX Fog2 heterozygous gonads have a decreased level of ectopic Sox9
Both the Ods mutation and the Wt1-Sox9 transgene cause complete female-to-male sex reversal by inducing a male-specific expression pattern of Sox9 in XX embryonic gonads (Bishop et al., 1999; Qin et al., 2003; Vidal et al., 2001). Remarkably, when crossed to the Fog2+/− females, the expression of the sex-reversed phenotype is inhibited and the F1 XX Fog2+/−Ods/+ or Wt1-Sox9 mice develop as fully fertile, phenotypic females. To determine the mechanism of this suppression, we compared the Sox9 expression in the Ods or Wt1-Sox9-positive E13.5 XX embryos that are either Fog2+/+ or Fog2+/− by whole-mount in situ hybridization. We observed no drastic reduction in the Sox9 staining in the Fog2+/− gonad compared to the Fog2+/+ control (Fig. 1, B-G).
It has been shown recently that testis differentiation is extremely sensitive to Sox9 levels and that a threshold of Sox9 expression should be reached to set the testis differentiation program in motion (Barrionuevo et al., 2006; Chaboissier et al., 2004; Qin and Bishop, 2005). We reasoned that a subtle difference in the level of expression might escape detection by in situ hybridization. To better measure the level of Sox9 expression we performed quantitative RT-PCRs. Real-time PCR analyses with RNA isolated from individual E12.5 urogenital ridges confirmed that the Sox9 level in Fog2+/− heterozygous gonads is approximately 50% of the Fog2+/+ controls (Fig. 1, H).
To evaluate the genetic consequences of the decrease in the Sox9 expression level we analyzed the expression of the Müllerian-inhibiting substance (Mis/Amh) gene, which has been suggested to be a direct target of Sox9 (Arango et al., 1999; Chaboissier et al., 2004; De Santa Barbara et al., 1998). As previously described, we observed a robust Mis expression in the E13.5 XX Ods/+ Fog2wt and Wt1-Sox9/+Fog2wt gonads (Figs. 2B and E); (Bishop et al., 1999; Vidal et al., 2001); however, in the transgenic XX Fog2+/− gonads Mis expression was undetectable (Figs. 2C and F), even when assessed by a sensitive RT-PCR assay (Fig. 2G).
Figure 2.
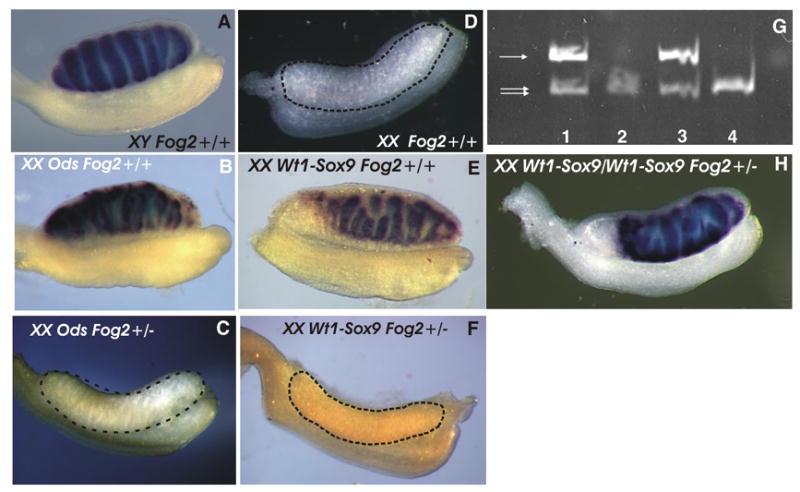
(A-F) Whole mount in situ hybridization analysis of the Mis expression in the E13.5 embryonic male (A), XX sex-reversed (B and E), XX Fog2 heterozygous (C and F) and XX (female) (D) gonads. Mis expression is absent from the control sample in (D); it is also not observed in the heterozygous Fog2 transgenic (Ods or Wt1-Sox9) gonads (C, F). (G) Semi-quantitative RT-PCR analysis of Mis gene expression in the XY (1), XX (2), XX Wt1-Sox9 (3) and XX Wt1-Sox9 Fog2+/− (4) E12.5 gonads. The positions of the bands corresponding to the Mis (arrow) and Hprt control (double arrow) PCR fragments are shown. (H) Whole mount in situ hybridization analysis of the Mis expression in the E14.0 double transgenic XX Wt1-Sox9/Wt1-Sox9 Fog2+/− gonad where robust Mis expression is apparent.
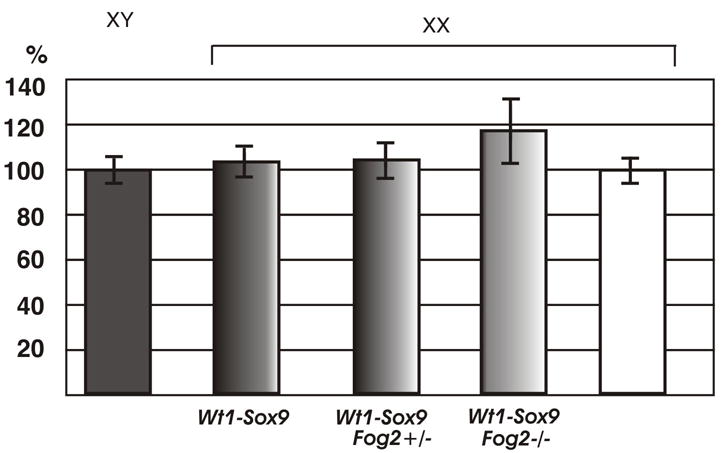
If a decrease in the Sox9 level is the sole reason for the suppression of sex reversal, merely increasing Sox9 expression should be sufficient to cause sex-reversal in the Fog2+/− animals. Since both XX Wt1-Sox9/Fog2+/− and XX OdsFog2+/− are fertile females, it was possible to obtain the homozygous Ods/Ods or Wt1-Sox9/Wt1-Sox9 animals by intercrossing them with the XY OdsFog2+/− or Wt1-Sox9Fog2+/− males, respectively. The double heterozygous, Ods/Wt1-Sox9, animals could similarly be obtained. We reasoned that having two copies of these genes should approximately double the ectopic Sox9 expression in the Fog2 heterozygous XX mice to the level sufficient for a sex-reversal (Fig. 1F). The analysis of the Ods/Ods, Wt1-Sox9/Wt1-Sox9 or mixed Ods/Wt1-Sox9 XX animals confirmed them to be the sex-reversed males irrespective of their Fog2 status as we have predicted (data not shown). While the XX Fog2+/− embryos harboring the single Ods or Wt1-Sox9 allele do not express Mis (Fig. 2C and F), Mis expression is robust in the gonads of the double-transgenic Wt1-Sox9Wt1-Sox9 Fog2+/− embryos (Fig. 2H). This experiment confirms that suppression of sex reversal in the Fog2 heterozygous females results solely from ~50% down-regulation of the Sox9 expression from the Ods or Wt1-Sox9 allele.
To confirm that Fog2+/−-mediated suppression of sex-reversal is not limited to Mis downregulation (which is a direct target of Sox9, (Arango et al., 1999) we examined the expression of other genes that are essential for male gonadal development. We could not detect the expression of Dhh, another marker of the Sertoli cell differentiation (Bitgood et al., 1996) as well as the Leydig cell-specific markers P450Scc and 3β-Hsd at any stage of development (Fig. 3 and data not shown). We conclude that the 50% reduction in the Sox9 expression suppresses female-to-male sex-reversal and expression of the male-specific genes, Mis, Dhh, P450Scc and 3β-Hsd. This data provides a molecular basis for the normal (i.e., not sex-reversed) phenotype of the XX Fog2+/− Ods or the Wt1-Sox9 XX Fog2+/− animals.
Figure 3.
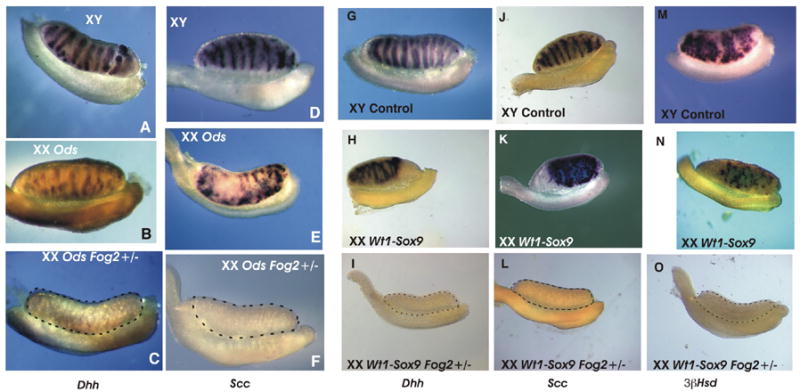
Whole mount in situ hybridization analysis of the Sertoli cell (A-C; G-I) and Leydig cell (D-F; J-O) gene expression in the E13.5-E14.5 gonads. XY control gonads/mesonephroi (A, D, G, J, M), XX sex-reversed gonads/mesonephroi (Ods, B, E; Wt1-Sox9, H, K, N) and XX Fog2 heterozygous gonads/mesonephroi (Ods, C, F; Wt1-Sox9, I, L, O) were hybridized with RNA probes to Desert hedgehog Dhh (A-C; G-I), P450scc (Scc)(D-F; J-L) and 3β-hydroxysteroid dehydrogenase/Δ[5]- Δ [4]-isomerase (3βHsd) (M-O). Marker gene expression is absent in the gonadal portion (encircled by a dotted line) of the XX Fog2+/− samples. All gonads within a set (A-C; D-F; G-I; J-L and M-O) are from the same experiment.
Ectopic Sox9 expression is lost in the absence of the functional GATA4/FOG2 complex
We and others have previously demonstrated that it is the GATA4/FOG2 partnership that is required to execute a number of critical decisions during organogenesis (Crispino et al., 2001; Tevosian et al., 2002). Specifically, fetuses homozygous for a targeted mutation (V205M) in Gata4, Gata4ki, that cripples the GATA4-FOG2 interaction, exhibit a profound and early block in gonadogenesis that is similar to the block noted in Fog2-null fetuses. Hence the Gata4ki allele allows a unique insight into the importance of the GATA4-FOG2 interaction for mammalian development (Crispino et al., 2001; Tevosian et al., 2002).
To determine whether the Sox9 expression driven by the Ods mutation or Wt1-Sox9 transgene requires the presence of the functional GATA4/FOG2 complex, we performed Sox9 in situ hybridization with the E12.0 Ods Gataki/ki mutants (Fig. 4). We have shown previously that up-regulation of the endogenous Sox9 expression in the XY fetal gonads requires GATA4-FOG2 interaction as Sox9 expression is blocked in either the Fog2 null or Gata4ki/ki embryos ((Tevosian et al., 2002); also Figs. 4B and F). The Sox9 expression in the heterozygous XX Ods Gata4ki/+ gonad (Fig. 4E) is comparable to that in the control Gata4+/+ sample (Fig. 4D) and is functionally sufficient to induce Mis expression and sex-reversal (data not shown); however, no Sox9 expression can be detected in either XY or XX Ods Gata4ki/ki homozygous mutants (Figs. 4C and G). Similarly, Sox9 expression was absent in the Wt1-Sox9 Gata4ki/ki, Ods Fog2-null, and Wt1-Sox9 Fog2-null E13.5 gonads in both sexes (Figs. 4H and I, and data not shown). We conclude that not only endogenous, but also ectopic Sox9 expression requires the presence of a functional GATA4/FOG2 complex.
Figure 4.
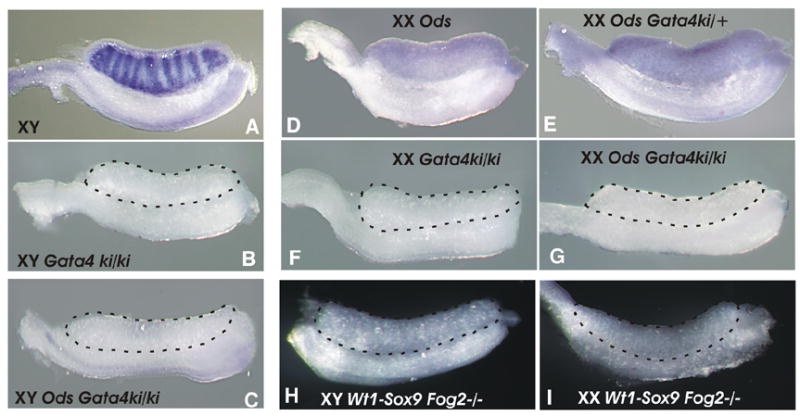
(A-G) Whole mount in situ hybridization analysis of the Sox9 expression in the XY (A-C) and XX (D-G) E12.0 gonads. Sox9 expression is detectable in the male control and Ods sex-reversed gonads (A, D, E); Sox9 expression is absent in the GATA4ki/ki homozygous gonads (B-C; F-G) even in the Ods mutants (XY, C; XX, G). (H-I) Whole mount in situ hybridization analysis of the Sox9 expression in the E13.5 XY (H) and XX (I) Wt1-Sox9 Fog2−/− gonads; Sox9 expression is absent.
Fog2 is equally expressed in embryonic gonads of both sexes
XY Fog2+/− animals express the wild-type levels of the Sry gene (Tevosian et al., 2002) and sufficient Sox9 (Fig. 1H; compare columns 1 and 2) to develop as normal fertile males; however, in the XX Fog2+/− mice the ectopic Sox9 expression caused by either the Ods mutation or Wt1-Sox9 transgene is suppressed below the threshold level (Fig. 1H; compare columns 2, 3 and 5 to columns 4 and 6). To exclude the possibility that this differential regulation is caused by the higher Fog2 expression in the XY (male) vs. XX (female) gonad we examined the early expression of Fog2 in mouse embryonic gonads. First, we evaluated Fog2 expression by analyzing the β-galactosidase (lacZ) marker expression in the Fog2-LacZ-ires-eGFP line of mice. In these animals the lacZ gene is incorporated (‘knocked-in’) into the Fog2 locus to allow LacZ expression as a fusion protein in-frame with the first 235 amino acids of the FOG2 protein. The Fog2-lacZ module is followed by an ires-eGFP cassette. This creates a null allele of the Fog2 gene (Fig. 5A). Our analysis of the embryonic gonads demonstrated that Fog2 is robustly expressed in both XY and XX as early as E11.5 (Fig. 5B-G). To further quantitate the Fog2 expression level we isolated cDNA from the XY and XX E12.5 gonads and performed qRT-PCR. We did not observe any difference in the Fog2 expression levels between the sexes at this stage; however, Fog2 heterozygous males express approximately 50% of the wild-type RNA level (Fig. 5H). Our data is consistent with the previously performed in situ hybridization analysis of Fog2 expression in mouse embryonic gonads (Ketola et al., 2002).
Figure 5.
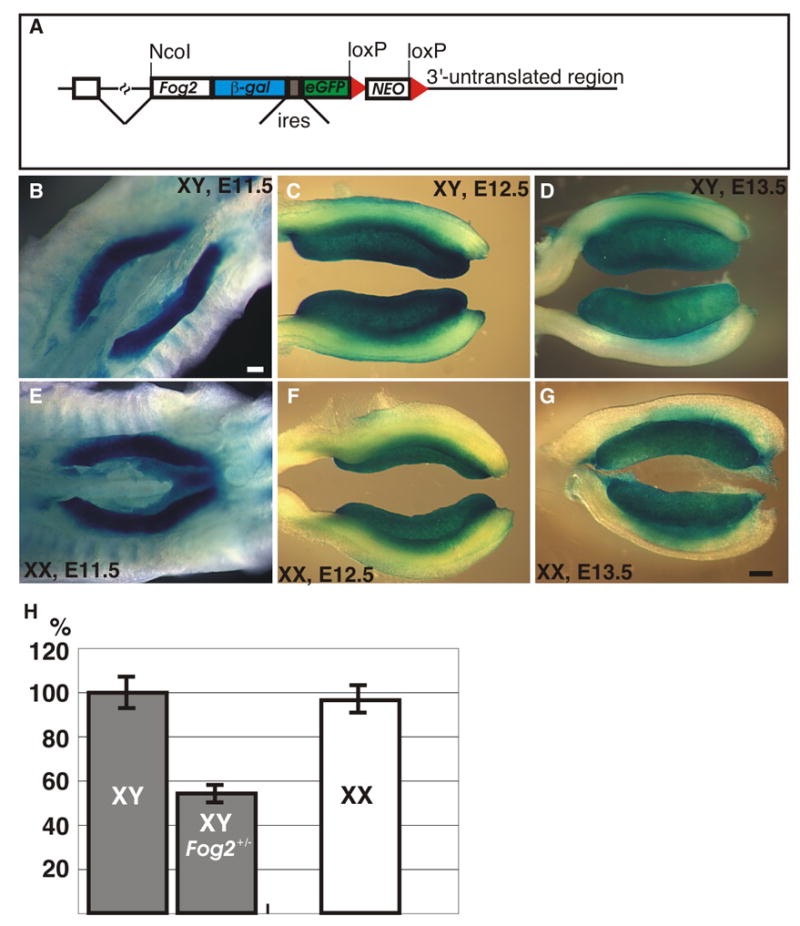
(A) Schematic representation of the Fog2-LacZ-eGFP targeted allele. The positive selection marker (neo) is flanked by the loxP sites (red triangles). The positions of the ires and the NcoI site are shown. (B-G) Whole-mount β-galactosidase staining of the gonads in the XY (B-D) and XX (E-G) Fog2-LacZ-eGFP embryos at E11.5 (B, E), E12.5 (C, F) and E13.5 (D, G). The scale bar is 200μm. (H) Taqman qRT-PCR analysis of the Fog2 gene expression in the XY (control and Fog2 heterozygous) and XX E12.5 gonads.
Transgenically-derived SOX9 is not acting in the catalytic fashion
We observed that despite carrying the Ods mutation or Sox9 over-expressing Wt1-Sox9 transgene, Fog2 null or GATA4ki/ki embryos (either XX or XY) fail to express detectable levels of Sox9 (Fig. 5 and data not shown). Although this result could be envisaged for the Ods mutation (see Introduction), this was clearly unexpected for the Wt1-Sox9. In the Wt1-Sox9 transgenics Sox9 expression is driven by the regulatory elements of the Wt1 locus encoded by a 620-kilobase mouse YAC (Vidal et al., 2001), the same elements that drive expression of the Wt1 gene itself. Wt1 expression is not affected by the Fog2 deficiency either in male or in female gonads (Tevosian et al., 2002; Fig. 6). ).
Figure 6.
Real-time PCR analysis (TaqMan) of Wt1 gene expression in the E12.5 embryonic gonads. Wt1 expression is not affected by the Wt1-Sox9 transgene presence or Fog2 deficiency.
It was also important to eliminate the possibility that the transgenic Sox9 in Wt1-Sox9 could act in a catalytic manner (analogous to Sry in the XY gonad) to activate the endogenous Sox9 in the pre-Sertoli cells of the XX gonad. In this case there could be a difference in the amount of Wt1-expressing vs. Sox9-expressing cells. To address this question we performed a qRT-PCR. The Wt1-driven Sox9 RNA could be distinguished from the endogenous Sox9 message by the presence of the SV40 polyadenylation sequence (Vidal et al., 2001). We detected no difference between the ectopic (SV40-containing) and the total Sox9 level in the XX Wt1-Sox9 E12.5 gonad (data not shown) confirming that Sox9 expression in the transgenic gonad is derived solely from the Wt1 transgene.
Fog2 haploinsufficiency leads to a decreased amount of Sox9-positive cells in the XY E12.5 gonads
Either Ods or the Wt1-Sox9 transgene is not sufficient to activate Sox9 expression in the absence of GATA4/FOG2 interaction. This suggested to us that GATA4/FOG2 interaction is specifically required either for the emergence of the Sertoli precursor cells or, more likely, for their subsequent survival. As Fog2 heterozygous animals express 50% of the control Sox9 level (Fig. 1) this should result in a corresponding decrease in the amount of SOX9-positive cells. To access the status of the SOX9-positive cells we performed a whole-mount staining of the E12.5 XY gonads with the SOX9 antibody. The amount of SOX9-positive cells in the E12.5 of the Fog2 heterozygous males decreases approximately two-fold (Fig. 7, A–B and E); however, overall cellular proliferation (mitotic index) does not appear to be affected (Fig. 7, C–D). To confirm that the reduction in SOX9-positive cells on E12.5 is not due to the earlier decrease in total cell numbers, we performed an analysis of processes that could affect the cell quantity, proliferation and apoptosis, on E11.5 No difference in phospho-H3 staining could be detected between the control and Fog2 heterozygous E11.5 samples (Fig. 8, A-B). Similarly, no changes in the amount of TUNEL-positive cells were observed between the control and Fog2 heterozygous samples, although apoptotic cells could be easily detected in the developing mesonephros (Fig. 8, C–D). We conclude that FOG2/GATA4 function is required to maintain precise numbers of Sertoli cells in the developing gonads.
Figure 7.
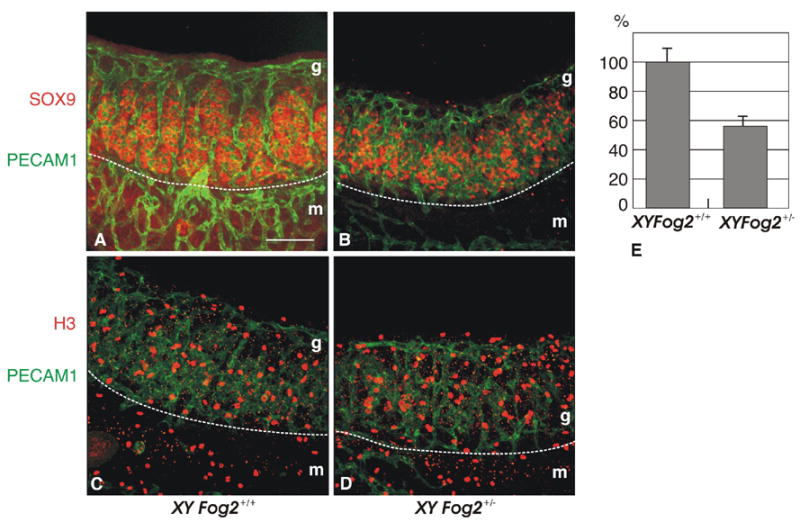
Whole-mount immunostaining of E12.5 XY gonads isolated from control (A, C) and Fog2 heterozygous (B, D) embryos. The gonads were stained with anti-PECAM1 (platelet/endothelial cell adhesion molecule 1) antibody that marks germ cells and vascular endothelial cells (green, A-D) and either anti-SOX9 antibody (red, A-B) or phosphorylated histone H3 (red, C-D). Fewer SOX9 positive cells were detected in the Fog2 heterozygous sample (B) compared to the control (A), while proliferation did not appear to be affected (C-D). The white dotted line indicated a boundary between the mesonephros (m) and gonad (g). The scale bar is 100μM.
(E). SOX9 positive cells were counted in three independent pairs of gonads of both genotypes. The total number of cells was counted per sample (50 planes); the amount of cells in the control is normalized to be 100%.
Figure 8.
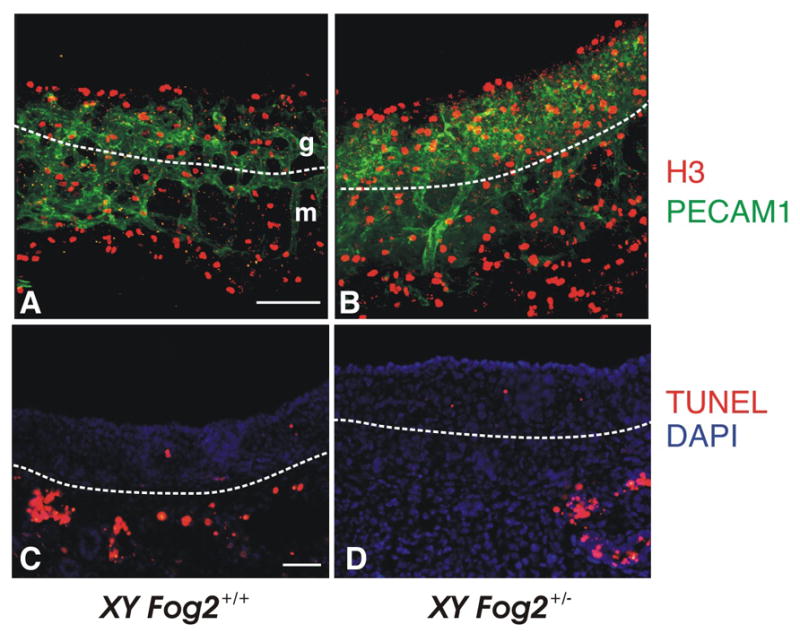
Comparison of the proliferation and apoptosis in the E11.5 XY gonads isolated from control (A, C) and Fog2 heterozygous (B, D) embryos. The gonads were stained with anti-PECAM1 antibody (green, A-B) and phosphorylated histone H3 (red, A-B). Frozen sections of gonad-mesonephros complex were stained to detect apoptotic cells/bodies (red; nick-end labeling, TUNEL) (C-D). No significant change in the proliferation (A-B) or apoptosis (C-D) are observed in the Fog2+/− samples compared to the control XY gonads. Apoptotic cells are easily detected around mesonephric tubules (arrows in D-F) as has been previously reported (e.g. (Allard et al., 2000; Kim et al., 2006). The white dotted line indicates the boundary between mesonephros (m) and gonad (g). The scale bar is 100 μM (A-B) and 50μm (C-D).
Discussion
SOX9/Sox9 is a key regulatory gene of the testis that is both necessary and sufficient for mammalian male sex differentiation. The regulation of Sox9 gonadal expression ensures precise (sex, organ and time) expression of this crucial sex-determining gene and remains the subject of considerable interest (Kanai and Koopman, 1999; Koopman et al., 2001). Better understanding of Sox9 regulation is essential to arrive at the genetic basis for mammalian testicular differentiation and function.
Examination of the Sox9 regulation in an Ods line of mice initially seemed to pin down the location for the elusive Sox9 gonadal cis-regulatory sequences. In Ods animals a tyrosinase minigene driven by the dopachrome tautomerase (Dct) promoter was randomly inserted ~1 Mb upstream of Sox9, additionally causing a 134-kb deletion of the adjacent sequence. Ods mice show female-to-male sex reversal, as well as microphthalmia with cataracts. The XX sex-reversal phenotype is accompanied by misexpression of Sox9 in the XX gonad, where Sox9 is usually repressed. It was originally proposed that the serendipitous deletion of the 134 kb region had removed the gonad-specific regulatory element(s) that would normally mediate the female-specific repression of Sox9, thus resulting in up-regulation of Sox9 and the consequent male development (Bishop et al. 2000). However, subsequent experiments have shown that the 134-kb deletion alone is insufficient to cause the sex reversal (Qin et al., 2004). A follow-up experiment recreated both the Ods deletion and the tyrosinase insertion (this time driven by its own promoter instead of the Dct); however, the Ods sex-reversal phenotype still was not observed (Qin et al., 2004).
In this study we did not focus on the analysis of the phenotype in wild-type XX Ods/+ animals as Ods-mediated sex reversal has been analyzed previously (Bishop et al., 1999; Qin et al., 2003). Specifically, it was reported that the animals on the pure FVB develop as 100% Ods/+ males. The contribution of genetic background on sex reversal was also examined; specifically, FVB/N XY Ods/+ carrier males have been crossed with several inbred strains; again, with the notable exception of the A/J strain, it was reported that F1 XX Ods/+ mice derived from these crosses develop as typical sex-reversed males (Qin et al., 2003). These crosses included the C57BL/6 and 129/Sv strains used in this (our) study. Even when crossed to the ICR (outbred) strain of mice 90% of the XX Ods/+ animals developed as males; the rest remained females (Bishop et al., 1999).
Our results are in agreement with the results reported by the Bishop’s group. For example, in this study external genitalia in 66 (95%) of the wild-type XX Ods/+ animals appeared male; only 4 mice (~6%) developed as females with microphtalmia. In stark contrast, all 53 (100%) of the XX Ods/+ Fog2 heterozygous animals developed as females (judged by their external genitalia).
We have not observed any animals with ambiguous genitalia or who are clear hermaphrodites; although sexual differentiation in the XX Ods/+ animals could be somewhat delayed, they develop testes and are sex reversed males. We also dissected gonads from ten of the adult XX Ods/+ animals obtained by crossing of the Ods/+ males with wild-type females (all animals were on the FVB/C57Bl/6/129 mixed background) and we performed a histological examination of the gonads; all ten of them had two small well-descended testes devoid of germ cells (Supplemental Figure 2). We have previously established an in vivo requirement for GATA4 and FOG2 in sexual differentiation. Fog2 null mouse fetuses or fetuses homozygous for a targeted mutation in Gata4 (Gata4ki) that cripples the GATA4-FOG2 interaction, exhibit a profound and early block in gonadogenesis in both sexes (Tevosian et al., 2002). We have now determined that Ods sex reversal (Bishop et al., 1999) as well as another previously described Sox9-dependent XX dominant sex reversal model, Wt1-Sox9 (Vidal et al., 2001), require a full complement of Fog2. On the contrary, a single fully functional allele of Gata4 provides sufficient amounts of the active GATA4/FOG2 complex to allow for ectopic XX Sox9 expression and a sex-reversal in these models. We conclude that FOG2 is a limiting factor for the formation of a functional GATA4/FOG2 complex in gonads. We also propose that Fog2 and/or Gata4 should be considered as potential modifiers for the Ods in addition to the one described previously (Qin et al., 2003).
Sox9 expression in the Ods mutant XX gonads of the Gata4ki/+ animals (Fig. 4E) (or in the XX Gata4ki Wt1-Sox9 transgenic, data not shown) appears unperturbed and is sufficient to cause sex-reversal; however, the complete absence of a wild type GATA4 protein in the homozygous GATA4ki/ki embryos (both XX or XY) blocks Sox9 expression (Fig. 4C and 4G) and the sex-reversal phenotype is suppressed even with the full complement of Fog2 being present. Hence, an interaction between GATA4 and FOG2 is required for the expression of the trangene-driven (Wt1-Sox9) or the mutant (Ods) Sox9.
GATA4ki/ki and FOG2−/− XY gonads initiate the male sex determination program by activating Sry expression, but Sry levels in FOG2−/− gonads are insufficient to induce the differentiation of Sertoli cells. Given that Sry expression is not properly activated and therefore testis differentiation is blocked at the earliest stage, it was previously impossible to determine whether GATA4/FOG2 complex have an in vivo role in testis determination independent of Sry regulation. The suppression of sex reversal that we now observe in the XX gonads has to rely on gene targets other than the Y chromosome-linked Sry gene. This observation is in accordance with several previous reports that propose the role for GATA/FOG factors in the regulation of multiple testis-specific genes (Martin et al., 2005; Robert et al., 2002; Tremblay and Viger, 2001). At this point we are unable to determine whether other genes (besides Sox9) require GATA4/FOG2 complex for their expression.
The expression from the wild-type (endogenous) Sox9 allele is shut down in the Gataki/ki or Fog2 mutant gonad (Tevosian et al., 2002), but we could not a priori predict whether the expression from the Ods allele of Sox9 will be lost as well. As we have now determined, Ods is not expressed in the Gata4/Fog2 mutant background; in fact, deletion of even a single functional Fog2 allele already interferes with the Ods expression. We conclude that inactivation of the GATA4/FOG2 complex equally affects the wild-type Sox9 and its Ods “version”. In other words, while the Ods mutation releases Sox9 from the negative control in XX gonads, it does not render Sox9 insensitive to GATA4/FOG2 regulation. This implies that GATA4/FOG2 similarly regulates both Sox9 and Ods and that this regulation is Sry–independent, although not necessarily direct (Fig. 9).
Figure 9.
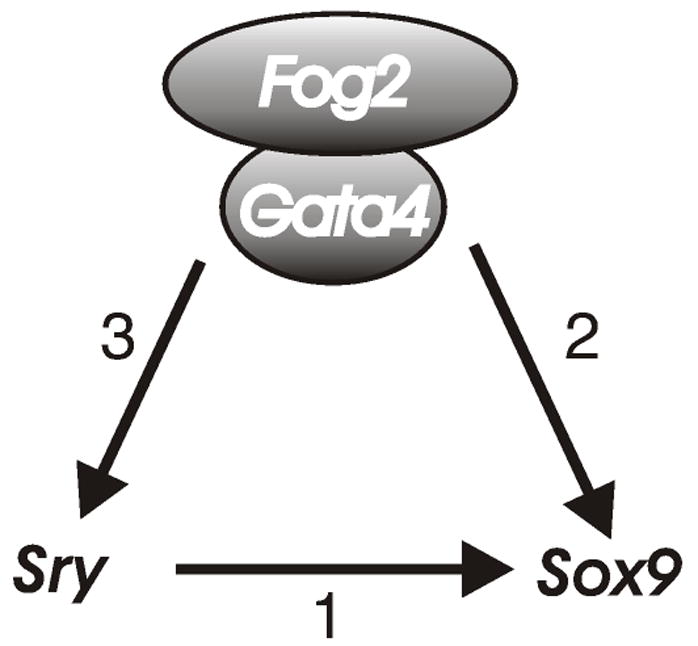
A combined action of SRY (1) and GATA4/FOG2 (2) complex is necessary for the male-specific activation of Sox9 gene expression. Normal Sry expression also requires GATA4/FOG2 complex (3) (Tevosian et al., 2002).
The 50% downregulation of expression from the Wt1-Sox9 transgene in the XX Fog2+/− mutant background and the complete loss of its expression in the GATA4ki/ki or FOG2 null gonads were quite unexpected. In the Wt1-Sox9 transgenics Sox9 is expressed from the Wt-1 regulatory element within a yeast artificial chromosome (YAC), faithfully mimicking gonadal expression of the endogenous Wt1 gene (Vidal et al., 2001). As our previous data indicated that Wt1 is expressed normally in the Fog2 null gonad (Tevosian et al., 2002), we expected the Wt1-driven Sox9 expression to be Fog2-independent. We cannot formally rule out the possibility that, in contrast to the endogenous Wt1 gene, regulatory elements present in the YAC require the full dosage of Fog2; however, that seems unlikely. Instead, we show that a reduction in the dosage of Fog2 gene expression leads to the concomitant decrease in the amount of SOX9 positive cells (Fig. 7, A–B) while the general proliferation and apoptosis in the gonad are not affected. Hence we propose that GATA4/FOG2 function is required to generate or maintain Sertoli cell precursors in the gonad. Although a direct, cell-autonomous activation of Sox9 gene transcription by GATA4 or GATA4/FOG2 complex cannot be excluded, we currently favor a model where GATA4/FOG2-induced signaling (e.g. through FGF9) ensures the survival of the Sertoli cell progenitors in the developing testis.
Experimental Procedures
Animals
The Odd sex (Ods) FVB animals were a kind gift of Paul Overbeek (Bishop et al., 1999); Wt1-Sox9 CBAxC57BL/6J transgenics were kindly provided by Andreas Schedl (Vidal et al., 2001). Transgenic lines were obtained by crossing these mice with Fog2 heterozygous and Gata4ki heterozygous animals (both mixed 129xC56BL/6 background); the generation and genotyping of Fog2- and Gata4-targeted animals have been previously described (Crispino et al., 2001; Tevosian et al., 2000). The Ods genotype was scored as Ods/+ or +/+ by examination of the eyes and by PCR analysis using primers specific for the transgenic construct 5’-CTGTCCAGTGCACCATCTGGACC -3’ and 5’-GATTACGTAATAGTGGTCCCTCAG-3’(Bishop et al., 1999). Wt-Sox9 transgenic animals were genotyped using PCR primers sWTp 5'-CATCCGAGCCGCACCTCATG-3' and SS2 5'-GCTGGAGCCGTTGACGCG-3’ as previously described (Vidal et al., 2001). Mice were genotyped for the presence of the Y chromosome by Sry or Zfy PCR as described previously (Tevosian et al., 2002) and also by the Southern blotting analysis using various fragments of the genomic Sry locus (p741, (Gubbay et al., 1990); generously provided by Kenn Albrecht and Eva Eicher) as probes. The mice were recorded as male or female after external and internal examination of the genitalia. The embryos were isolated and staged as previously described (Tevosian et al., 2002).
Generation of the reporter knock-in into the Fog2 locus
All genomic DNA corresponding to various regions of the Fog2 gene was obtained from the pBSK KSII (Stratagene) subclones of the λFixII 129 mouse genomic library (Stratagene). The NcoI-XbaI β-galactosidase gene from pSDKlacZpA (a kind gift of Janet Rossant) was subcloned into pBSK KSII (this cloning strategy does not retain the polyadenylation signal of the LacZpA cassette). A 2.6kb SalI-NcoI fragment containing a 5’ homology region of the Fog2 gene was cloned in-frame with the LacZ gene. Next, the ires-EGFP-SV40pA fragment from pIRES2-EGFP (Clontech) was cloned in the XbaI site of this vector. The resulting Fog2-β-gal-ires-EGFP cassette was transferred into the pTKLNCL targeting vector (Tevosian et al., 2000) to generate pTKLNCL-5’. The NotI fragment containing the 5.1kb of the Fog2 3’ region was introduced into the NotI site of the pTKLNCL-5’ to generate the targeting vector. The resulting plasmid was isolated by CsCl centrifugation, linearized with KspI and electroporated into the CJ7 ES cells. The neo/tk-selected ES clones have been genotyped by Southern blotting analysis. The correctly targeted ES cells were injected into the C57BL/6 blastocysts. The Fog2-LacZ-ires-eGFP strain was maintained on the mixed, C57BL/6x129 background.
Whole mount in situ hybridization
Embryos of various stages were dissected from the uterus and their internal organs were removed to expose the gonads. Embryos were fixed with 4% paraformaldehyde (PFA) in 1xPBS at 40C overnight. Further processing of embryos and in situ hybridization analysis were carried out essentially as described (Wilkinson, 1992). Sox9, Mis, Scc, Dhh and 3βHsd dig-labeled RNA probes have been previously described (Tevosian et al., 2002). Gonads were photographed and images were processed and assembled as previously described (Adameyko et al., 2005).
Quantitative RT-PCR analysis
Individual gonad/mesonephros complexes were dissected in PBS from E11.5-E13.5 embryos and transferred in RNAlater solution (Ambion). RNA was isolated with RNeasy Mini kit (Qiagen) in 30μl of RNAse-free TE buffer. RNA samples were treated with RQ1 DNase (Promega) for 30 min and RNA was extracted with phenol:chloroform and ethanol precipitated according to standard procedures. Each sample was divided into two aliquots, one of which was reverse transcribed using the SuperScript First Strand Synthesis System (Invitrogen), following the manufacturer’s instructions. The second aliquot was used as a control (RT-PCR without reverse transcription) to identify samples with DNA contamination. All real time PCR assays were carried out using TaqMan Universal PCR Master Mix (Applied Biosystems). The PCR reactions contained a) 25 ng of cDNA, b) the gene specific primers at a final concentration of 1 μM each and c) the Taqman probe at a final concentration of 0.25 μM. The assays were run under standard Taqman conditions on the ABI 7500 instrument. A standard curve for each gene was generated using serial dilutions of cDNA. Relative expression levels of each sample were determined in the same run and were expressed as the ratio of the RNA amount (of interest) to the amount of a control RNA (β-actin). TaqMan reactions were performed in duplicates and the experiments were repeated independently at least three times (for at least three samples). Gene-specific primers and probes were designed using the Primer Express software (Perkin Elmer Life Sciences), namely qRT-PCR primers: β-actin; 5’-ACGGCCAGGTCATCACTATTG-3’ and 5’-CAAGAAGGAAGGCTGGAAAAGA-3’, with hybridization probe 5’FAM- CAACGAGCGGTTCCGATGCCC-BHQ3’ Fog2; 5’-GCAAGTCCTGTGGCATCTG-3’ and 5’-CTCGTTGCCTCCCACTACAGTA-3’, hybridization probe 5’FAM- AGCGGAACCTGCAAGCCCATTTG-BHQ3’ Wt1; 5’-AGGACACGACTGTGGATCTACATC -3’ and 5’-TTCCGGCAAACCTGATAGGA -3’, hybridization probe 5’FAM- TCCAAGACAGCACACCTGATTGACTGC-BHQ3’ Sox9; 5’-CAAGCGGAGGCCGAAGA -3’ and 5’-CAGCTTGCACGTCGGTTT -3’, hybridization probe 5’FAM-CCACCCACCACTCCCAAAACCGAC-BHQ3’ RT-PCRs were performed for 30 cycles (95°C for 1 minute, 55°C for 1 minute 30 seconds and 72°C for 3 minutes), and 1 cycle for 5 minutes at 72°C RT-PCR primers for semi-quantitative analysis of the Mis gene expression were 5’-GCAGTTGCTAGTCCTACATCTGGCT -3’ and 5’-TGGAGGCTCTTGGAACTTCAGCAA -3’. SV40 primers were 5’-TGAGTTTGGACAAACCACAAC -3’ and 5’-CCCCCTGAACCTGAAACATA-3’. For an analysis of the Fst and Bmp2 gene expression, quantitative Real Time RT-PCR was performed using a PCR SYBR Green I Kit (Applied Biosystems) according to the manufacturer's instructions in a 7500 Fast RT-PCR system machine. A standard amplification protocol was established for both genes. Calibration curves were generated using dilutions of a corresponding gel-purified RT-PCR product; calibration points for serial dilutions were from 106 to 102 copies. The value for each gene was normalized to the Gapdh gene value and these ratios were compared between XX controls and Ods/Wt1-Sox9 Fog2 heterozygous samples. The gene copy number was calculated with ABI SDS Software Version 1.3.1. Values from five independent experiments were compared; standard deviation and a Student's t-test were calculated using Excel (Microsoft). Gene-specific primers were designed using the Primer3 software on the Web (Rozen and Skaletsky, 2000); Fst primers were 5’-AGAGGTCGCTGCTCTCTCTG-3’ and 5’-AGCTTCCTTCATGGCACACT-3’; Bmp2 primers were 5’-CGACGGAACATTCTCCAAAT-3’ and 5’-ATTACGGGATTCTCGGAGGT-3’.
β-Galactosidase assay
Embryos were fixed and stained using X-gal essentially as previously described (Adameyko et al., 2005). The staining was continuously monitored until a satisfactory color development was achieved (2 to 5 hr). Embryos were then fixed overnight in 4% paraformaldehyde in PBS and photographed as previously described (Adameyko et al., 2005).
Whole-mount immunostaining and TUNEL assay
Whole-mount immunohistochemical analysis was performed as described previously (Albrecht and Eicher, 2001; Kim et al., 2006). Briefly, E11.5-E12.5 gonad-mesonephros complexes were fixed overnight at 4°C in 4% paraformaldehyde, followed by a 24 hour incubation in a blocking buffer (1% BSA, 0.1% saponin, 0.02% sodium azide in PBS) at 4°C. Samples were incubated with primary antibodies for 24 hours, washed and incubated for another 24 hours with secondary antibodies. Primary antibodies were rabbit anti-SOX9 (1:1000), rat anti-PECAM1 (BD Biosciences, 1:500) and rabbit anti-phosphorylated histone H3 (Cell Signaling; 1:250). Secondary antibodies used for visualization were anti-rabbit and anti-rat Alexa Fluor-488 and -555 conjugated (Invitrogen; 1:750). Fluorescently labeled samples were mounted in Slowfade (Invitrogen). Images were obtained using a Leica TCS-NT laser-scanning confocal microscope, and assembled using Adobe Photoshop (Adobe). Apoptotic cells were detected in frozen sections of the E11.5-E12.5 gonads. Samples were frozen in the OCTcompound (VWR) and sectioned on the cryostat (Leica) to obtain 10μ sections. Apoptotic cells were detected by terminal deoxynucleotide UTP nick-end labeling (TUNEL) using an In Situ Cell Death Detection kit (Roche Biosciences) according to the manufacturer’s instructions. Nuclei were counterstained with Vectashield with DAPI (Vector).
Supplementary Material
Supplemental Figure 1. (A). Dissected ovaries from the adult control (left) and Ods/Fog2+/− (right) females. (B-E) Ovaries in (A) have been fixed, embedded in paraffin, sectioned and stained with hematoxylin/eosin. Control (B, D) and Ods/Fog2+/− (C, E) ovaries are shown. Magnification 40X (B-C) and 100X (D, E).
Supplemental Figure 2. (A). Dissected testes from the adult control (right) and XX Ods (left) males. (B-C) Testes in (A) have been fixed, embedded in paraffin, sectioned and stained with hematoxylin/eosin. XX Ods (B) and normal (C) testes are shown. Magnification 100X (B-C).
Acknowledgments
We thank Paul Overbeek for providing the Ods mice and Andreas Schedl for providing the Wt1-Sox9 animals. We also thank Kenn Albrecht for his advice throughout this work and for the critical reading of the manuscript. This work was supported by a grant to S.G.T from the National Institutes of Health (NIH NICHD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adameyko II, Mudry RE, Houston-Cummings NR, Veselov AP, Gregorio CC, Tevosian SG. Expression and regulation of mouse SERDIN1, a highly conserved cardiac-specific leucine-rich repeat protein. Dev Dyn. 2005;233:540–52. doi: 10.1002/dvdy.20368. [DOI] [PubMed] [Google Scholar]
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-sertoli cells and sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Allard S, Adin P, Gouedard L, di Clemente N, Josso N, Orgebin-Crist MC, Picard JY, Xavier F. Molecular mechanisms of hormone-mediated Mullerian duct regression: involvement of beta-catenin. Development. 2000;127:3349–60. doi: 10.1242/dev.127.15.3349. [DOI] [PubMed] [Google Scholar]
- Arango NA, Lovell BR, Behringer RR. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99:409–19. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G. Homozygous Inactivation of Sox9 Causes Complete XY Sex Reversal in Mice. Biol Reprod. 2006;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison WR, Behringer RR, Overbeek PA. A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet. 1999;26:490–4. doi: 10.1038/82652. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Capel B. The battle of the sexes. Mech Dev. 2000;92:89–103. doi: 10.1016/s0925-4773(99)00327-5. [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- Crispino JD, Lodish MB, Thurberg BL, Litovsky SH, Collins T, Molkentin JD, Orkin SH. Proper coronary vascular development and heart morphogenesis depend on interaction of GATA-4 with FOG cofactors. Genes Dev. 2001;15:839–44. doi: 10.1101/gad.875201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol Cell Biol. 1998;18:6653–65. doi: 10.1128/mcb.18.11.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–30. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–50. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hiramatsu R, Matoba S, Kidokoro T. From SRY to SOX9: mammalian testis differentiation. J Biochem (Tokyo) 2005;138:13–9. doi: 10.1093/jb/mvi098. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Koopman P. Structural and functional characterization of the mouse Sox9 promoter: implications for campomelic dysplasia. Hum Mol Genet. 1999;8:691–6. doi: 10.1093/hmg/8.4.691. [DOI] [PubMed] [Google Scholar]
- Ketola I, Anttonen M, Vaskivuo T, Tapanainen JS, Toppari J, Heikinheimo M. Developmental expression and spermatogenic stage specificity of transcription factors GATA-1 and GATA-4 and their cofactors FOG-1 and FOG-2 in the mouse testis. Eur J Endocrinol. 2002;147:397–406. doi: 10.1530/eje.0.1470397. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P. Sry and Sox9: mammalian testis-determining genes. Cell Mol Life Sci. 1999;55:839–56. doi: 10.1007/PL00013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P, Bullejos M, Bowles J. Regulation of male sexual development by Sry and Sox9. J Exp Zool. 2001;290:463–74. doi: 10.1002/jez.1089. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R, Canning C, Sekido R. Sex-determining genes in mice: building pathways. Novartis Found Symp. 2002;244:4–18. 18–22, 35–42, 253–7. [PubMed] [Google Scholar]
- Martin LJ, Taniguchi H, Robert NM, Simard J, Tremblay JJ, Viger RS. GATA factors and the nuclear receptors, steroidogenic factor 1/liver receptor homolog 1, are key mutual partners in the regulation of the human 3beta-hydroxysteroid dehydrogenase type 2 promoter. Mol Endocrinol. 2005;19:2358–70. doi: 10.1210/me.2004-0257. [DOI] [PubMed] [Google Scholar]
- McElreavey K, Vilain E, Abbas N, Herskowitz I, Fellous M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc Natl Acad Sci U S A. 1993;90:3368–72. doi: 10.1073/pnas.90.8.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer D, Kist R, Dewar K, Devon K, Lander ES, Birren B, Korniszewski L, Back E, Scherer G. Campomelic dysplasia translocation breakpoints are scattered over 1 Mb proximal to SOX9: evidence for an extended control region. Am J Hum Genet. 1999;65:111–24. doi: 10.1086/302455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Bishop CE. Sox9 is sufficient for functional testis development producing fertile male mice in the absence of Sry. Hum Mol Genet. 2005;14:1221–9. doi: 10.1093/hmg/ddi133. Epub 2005 Mar 24. [DOI] [PubMed] [Google Scholar]
- Qin Y, Kong LK, Poirier C, Truong C, Overbeek PA, Bishop CE. Long-range activation of Sox9 in Odd Sex (Ods) mice. Hum Mol Genet. 2004;13:1213–8. doi: 10.1093/hmg/ddh141. [DOI] [PubMed] [Google Scholar]
- Qin Y, Poirier C, Truong C, Schumacher A, Agoulnik AI, Bishop CE. A major locus on mouse chromosome 18 controls XX sex reversal in Odd Sex (Ods) mice. Hum Mol Genet. 2003;12:509–15. doi: 10.1093/hmg/ddg045. [DOI] [PubMed] [Google Scholar]
- Robert NM, Tremblay JJ, Viger RS. Friend of GATA (FOG)-1 and FOG-2 differentially repress the GATA-dependent activity of multiple gonadal promoters. Endocrinology. 2002;143:3963–73. doi: 10.1210/en.2002-220280. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Humana Press; Totowa, NJ: 2000. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development. 2002;129:4627–34. doi: 10.1242/dev.129.19.4627. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–39. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS. GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology. 2001;142:977–86. doi: 10.1210/endo.142.3.7995. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, de RD, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–7. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Mullerian inhibiting substance promoter. Development. 1998;125:2665–75. doi: 10.1242/dev.125.14.2665. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–20. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Clarke TR, Lane AH, Wang X, Donahoe PK. Endogenous expression of Mullerian inhibiting substance in early postnatal rat sertoli cells requires multiple steroidogenic factor-1 and GATA-4-binding sites. Proc Natl Acad Sci U S A. 2000;97:1624–9. doi: 10.1073/pnas.97.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderle VM, Critcher R, Hastie N, Goodfellow PN, Schedl A. Deletion of long-range regulatory elements upstream of SOX9 causes campomelic dysplasia. Proc Natl Acad Sci U S A. 1998;95:10649–54. doi: 10.1073/pnas.95.18.10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. (A). Dissected ovaries from the adult control (left) and Ods/Fog2+/− (right) females. (B-E) Ovaries in (A) have been fixed, embedded in paraffin, sectioned and stained with hematoxylin/eosin. Control (B, D) and Ods/Fog2+/− (C, E) ovaries are shown. Magnification 40X (B-C) and 100X (D, E).
Supplemental Figure 2. (A). Dissected testes from the adult control (right) and XX Ods (left) males. (B-C) Testes in (A) have been fixed, embedded in paraffin, sectioned and stained with hematoxylin/eosin. XX Ods (B) and normal (C) testes are shown. Magnification 100X (B-C).


