Summary
It was recently shown that the structure of the fluorophore attached to the acyl chain of phosphatidylcholine analogs determines their mechanism of transport across the plasma membrane of yeast cells (Elvington et al., J. Biol Chem. 280:40957, 2005). In order to gain further insight into the physical properties of these fluorescent phosphatidylcholine (PC) analogs, the rate and mechanism of their intervesicular transport was determined. The rate of spontaneous exchange was measured for PC analogs containing either NBD (7-nitrobenz-2-oxa-1,3-diazol-4-yl), Bodipy FL (4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene), Bodipy 530 (4,4-difluoro-5,7-diphenyl-4-bora-3a,4a-diaza-s-indacene), or Bodipy 581 (4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene) attached to a five or six carbon acyl chain in the sn-2 position. The rate of transfer between phospholipid vesicles was measured by monitoring the increase in fluorescence as the analogs transferred from donor vesicles containing self-quenching concentrations to unlabeled acceptor vesicles. Kinetic analysis indicated that the transfer of each analog occurred by diffusion through the water phase as opposed to transfer during vesicle collisions. The vesicle-to-monomer dissociation rate constants differed by over four orders of magnitude: NBD-PC (kdis = 0.115 s−1; t½ = 6.03 s); Bodipy FL-PC (kdis = 5.2 × 10−4; t½ = 22.2 min); Bodipy 530-PC (kdis = 1.52 × 10−5; t½ = 12.6 h); and Bodipy 581-PC (kdis = 5.9 × 10−6; t½ =32.6 h). The large differences in spontaneous rates of transfer through the water measured for these four fluorescent PC analogs reflect their hydrophobicity and may account for their recognition by different mechanisms of transport across the plasma membrane of yeast.
Keywords: phosphatidylcholine, phospholipid, NBD, Bodipy, fluorescence, intervesicular transfer
INTRODUCTION
Fluorescent labeled lipid analogs have proven to be useful tools in the study of transport and trafficking of phospholipids in a variety of cell types (reviewed in [1–4]). Phospholipid reporter molecules can be synthesized using a variety of head groups, and are typically constructed by replacing one of the naturally occurring fatty acids with a shortened acyl chain with a covalently attached fluorophore. The shortened acyl chain contained in these reporters produces a more water soluble molecule that can be readily introduced into cellular membranes. The fluorescent moiety allows for tracking of the lipid as it is internalized and distributed throughout a cell.
In recently published work, we have demonstrated that the fluorescent moiety itself is sufficient to determine the mechanism of uptake of phosphatidylcholine across the plasma membrane of Saccharomyces cerevisiae [5]. In this study, we found that the previously defined mechanism of internalization of the PC analog labeled with the fluorescent NBD1 group (NBD-PC) differed from that responsible for internalization of two other fluorescent PC analogs (Bodipy 530-PC and Bodipy 581-PC). Flip of another analog tested (Bodipy FL-PC) was dependent upon the same gene products as NBD-PC, but deletion of these genes did not reduce Bodipy FL-PC uptake to the same extent as that of NBD-PC.
In an effort to gain further insight into the physical-chemical properties of the fluorophores that allow these phosphatidylcholine analogues to be distinguished by plasma membrane transporters, we measured their rates of spontaneous, intervesicular transfer between phospholipid vesicles. Differences in biophysical properties of NBD-PC and Bodipy-FL PC have already been demonstrated in several studies involving model membranes. Analyses of the distribution of the NBD group within the membrane indicate that the fluorophore is much more likely to exist at the membrane-water interface as opposed to being imbedded in the bilayer [6, 7]. This holds true even if the length of the acyl chain containing the NBD group is increased from 6 to 12, demonstrating the degree to which the polar nature of NBD plays a part in the overall characteristics it imparts to the lipid [8]. Similar experiments involving the Bodipy fluorophore indicate that it is much more likely than NBD to be buried within the membrane [9]. This difference in degree of imbedding of the fluorophores may contribute to the differences in transfer properties observed for NBD-PC and Bodipy FL-PC. While spontaneous transfer of NBD-PC from donor to acceptor vesicles occurs within a few seconds (t½ = 2 s at 25°C [10]), transfer of Bodipy FL-PC takes several minutes (t½ = 350 s at 22°C [11]). Additionally, the rate of spontaneous transbilayer movement (flip-flop) of Bodipy-FL was found to be close to that observed with the endogenous phospholipid dimyristoyl PC. These data suggest that more hydrophobic PC derivatives may be better suited to studies of PC transport, since their flip-flop rates and distribution in the membrane are closer to that of endogenous PC.
The work presented here expands upon these previous observations to give comparisons of several biophysical characteristics of NBD-PC, Bodipy FL-PC, Bodipy 530-PC, and Bodipy 581-PC. The self-quenching characteristics of each fluorophore-PC molecule were examined and used to monitor the type and rate of transfer between donor and acceptor vesicles. While transfer of each analog was found to occur by diffusion through the water, the analogs differed in their transfer rates between vesicles by more than 4 orders of magnitude. Transfer of NBD-PC occurred in seconds while transfer of the more hydrophobic Bodipy derivatives took several hours, indicating the structure of the fluorophore present on the acyl chain is sufficient to significantly alter the rate of bilayer dissociation of phosphatidylcholine analogs.
MATERIALS AND METHODS
Materials
All reagents, unless otherwise noted, were purchased from Sigma. POPC and NBD-PC were purchased from Avanti Polar Lipids. All other lipids, including Bodipy FL-PC, Bodipy 530-PC, and Bodipy 581-PC, were purchased from Molecular Probes. Stocks of the four fluorescent phospholipids (5 mM) were prepared by solubilization of the dried phospholipids in chloroform. Phospholipids were stored at −20 °C and periodically monitored for purity by thin-layer chromatography, and repurified by preparative thin-layer chromatography as necessary. Fluorescent phospholipid concentrations were determined by absorbance in methanol at 466 nm for NBD-PC (ε=22,000), 503 nm for Bodipy FL-PC (ε=80,000), 534 nm for Bodipy 530-PC (ε=64,000), and 582nm for Bodipy 581-PC (ε=124,000). Molar absorptivity values were provided by Molecular Probes.
Vesicle Preparation
Large unilamellar vesicles with various concentrations of fluorescent PC were prepared by extrusion [12]. POPC was mixed with one of the fluorescent PCs to achieve the following approximate molar percentages: 0.1, 0.3, 0.5, 0.75, 1.0, and 2.0% for the Bodipy lipids. POPC was replaced by NBD-PC to reach a relative concentration of 2.0, 3.0, 5.0, 7.0, 10, and 20 molar percent. These mixtures were then dried under nitrogen and desiccated to remove trace solvent. HEPES buffered saline (HBS; 10mM HEPES, 0.9% NaCl, pH 7.4) was added to each aliquot and vortex mixed to reach an approximate concentration of 1 mM total phospholipid. The phospholipid mixtures were extruded 10 times through two stacked polycarbonate filters (0.1 μm pore size) to produce large unilamellar vesicles. After preparation, the concentration of phospholipid present in each aliquot of vesicles was determined by phosphate assay [13]. The exact concentration of fluorescent PC present in each aliquot was determined by absorbance in methanol.
Fluorescence Measurements
Fluorescent measurements were performed in an SLM 8000C fluorometer using the “slow time course” mode with excitation and emission slits set to 4 mm. Solutions in the cuvette were stirred continuously, and temperature was kept constant at 23 °C. Samples were excited and emission detected using the following wavelengths: 475/530 nm for NBD-PC, 500/510 nm for Bodipy FL-PC, 530/550 nm for Bodipy 530-PC, and 580/590 nm for Bodipy 581-PC.
Standard Curves and Kinetic Measurements
Standard quenching curves were generated for each fluorescent-PC analog by measuring the fluorescence of 10 μM total phospholipid containing various molar percentages of fluorescent-PC in POPC. For kinetic experiments, 10 nmol of donor vesicles containing fluorescent-PC at quenching concentrations (~2.0% Bodipy-PC’s or ~20% NBD-PC) was added to 1.0 mL HBS buffer in the fluorescence cuvette. After equilibration, a 20-fold excess of acceptor vesicle was added and the transfer of fluorescent PC from donor to acceptor vesicles was monitored as an increase in fluorescence as a function of time.
Theoretical Basis for Data Analysis
Concentration-dependent fluorescence quenching
Although the mechanism of concentration-dependent or self quenching of the NBD and Bodipy fluorophores is not well understood [14], we found that a modification of the Stern-Volmer equation based on fluorescence quenching upon monomer-monomer and monomer-dimer interactions [15]
| (1) |
adequately described the relationship of fluorescence intensity to fluorophore concentration presented in Figure 2
Figure 2. Quenching curves relating fluorescence to concentration of fluorophore within vesicles.
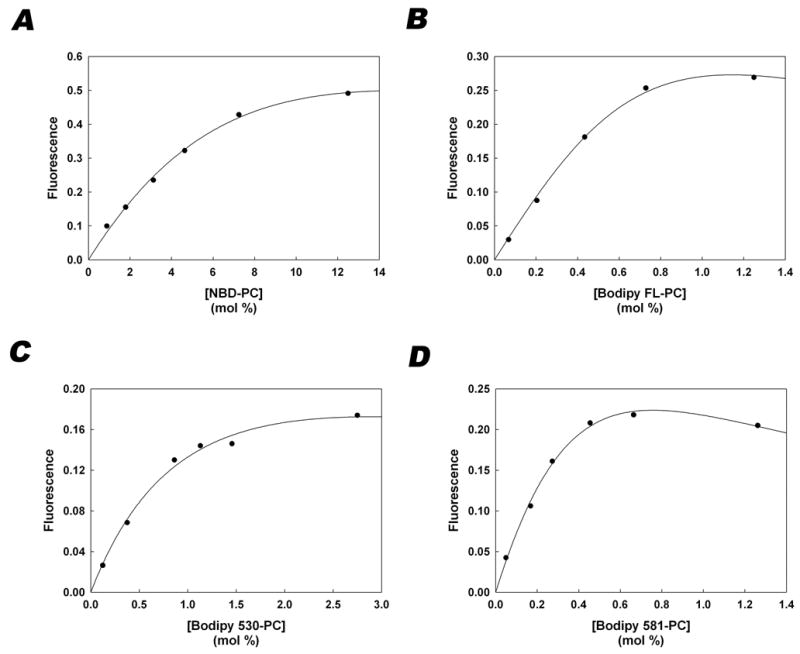
Plots of fluorescence versus concentration of NBD-PC (A), Bodipy FL-PC (B), Bodipy 530-PC (C), and Bodipy 581-PC (D). Regression analysis described in Theoretical Basis for Data Analysis was used to find the values of Ko, Km, and Kd that gave the best fit to the data. The symbols represent the measured data and the solid line was generated by substituting the following K values into equation 3 (NBD-PC: Ko=0.97, Km=6.12, Kd=44.4; Bodipy FL-PC: Ko=4.77, Km=4.67 × 10−7, Kd =7630; Bodipy 530-PC: Ko=2.40, Km=61.3, Kd=1820 ; Bodipy 581-PC: Ko=8.09, Km=97.5, Kd=17400)
Fo is the fluorescence intensity at low membrane concentrations of fluorophore in the absence of self-quenching and F is the fluorescence intensity at higher fluorophore concentrations for which monomer-monomer and monomer-dimer interactions result in self-quenching. Bv is the amount of fluorophore in the membrane and Lv is the amount of vesicular phospholipid. Km and Kd are empirical constants that relate monomer-monomer and monomer-dimer quenching interactions, respectively. At low concentrations, the fluorescence intensity is directly related to the amount of fluorophore such that:
| (2) |
Substituting Equation 2 into Equation 1 and rearranging results in the following equation that predicts the measured fluorescence intensity as a function of the amount of fluorophore.
| (3) |
The data of fluorescence as a function of fluorophore membrane concentration (Figure 2) was fit to Equation 3 using the Sigmaplot regression program to determine the values of Ko, Km, and Kd for each of the fluorescent-labeled PCs.
Kinetic analysis of monomer transfer between vesicle membranes
A theoretical relationship describing the transfer of slightly water soluble amphiphiles between membrane vesicles has been derived previously [16]. When the donor and acceptor vesicles have the same composition (the small amounts of fluorescent PC in the donors are assumed to make insignificant alterations of the association and dissociation rate constants), the rate equation simplifies to Equation 4 which describes the amount of fluorophore in the acceptor vesicles (Ba) as a function of time (t).
| (4) |
Bt is the total amount of fluorescent PC initially incorporated into the donor vesicles. Ld and La are the amounts of phospholipid in the donor and acceptor vesicles, respectively. k is the dissociation rate constant from the vesicles, and α is the fraction of the fluorophore residing in the outer leaflet of the donor vesicles which was assumed to be 0.5. Movement or flip-flop of the fluorophore from the inner to outer leaflet is assumed to be slow relative to the rates of transfer between vesicles.
According to the experimental protocol, at time zero, the fluorescence intensity will emanate solely from the donor vesicles. Following the addition of excess acceptor vesicles, the fluorescent PC begins to reequilibrate, and the fluorescence of the acceptor vesicles (Fa) increases while the fluorescence of the donor vesicles (Fd) either increases or decreases depending on the concentration of fluorescent PC. The measured change in fluorescence (Ft) is the sum of Fd and Fa. Assuming flipflop is slow relative to the rate process, changes in fluorescence will arise from the change in fluorophore concentration in the outer leaflets of the donor and acceptor vesicles.
Equation 3 can be modified to predict the fluorescence intensity associated with the outer leaflet of the acceptor vesicles (Fao), the outer leaflet of the donor vesicles (Fdo), and the inner leaflet of the donor vesicles (Fdi) as follows,
| (5) |
| (6) |
and
| (7) |
and the predicted measured fluorescence equals their sum:
| (8) |
The subscript a refers to acceptor vesicles and ao to the outer leaflet of the acceptor vesicles , d, do, and di refer to donor vesicles, outer leaflet of the donor vesicles and inner leaflet of the donor vesicles, respectively. Fao and Fdo will vary during the transfer process while Fdi will remain constant since the fluorophore in the inner leaflet is assumed to not flip-flop to the outer leaflet at a significant rate relative to the transfer process. These equations are based on the assumption that the concentration dependent quenching for each bilayer leaflet is quantitatively similar to the whole membrane.
Equation 4 was used to predict Bao as a function of time and since
| (9) |
and
| (10) |
the change in Ftheor as a function of time was calculated from Equations 5–8 by substituting the fluorophore specific values for the constants Ko, Km, and Kd, and the Sigmaplot regression analysis program was used to determine the best fit value for the dissociation rate constants, k. The half-time for equilibration was calculated from the relationship:
RESULTS
NBD- and Bodipy-labeled phosphatidylcholine analogs self-quench in phospholipid bilayers
The fluorescence of the NBD- and Bodipy-labeled phosphatidylcholine analogues (Figure 1) was measured as a function of their mole fraction in POPC vesicles (Figure 2). As observed previously for NBD-PC [17], the fluorescence of each of the PC analogues deviated from linearity, reached a plateau and ultimately decreased as the mole fraction of the analogue was increased. Although the mole fraction at which the quenching interactions were observed differed between the analogues, the degree of self-quenching was sufficient to measure changes in fluorescence as the fluorescent-PC analogues equilibrated into acceptor vesicles. In the absence of detailed knowledge of the mechanism responsible for the quenching interactions for each of the fluorophores, the data were fit to a theoretical relationship that predicts the dependence of fluorescence on the concentration of freely fluorescing monomers and completely quenched monomer-monomer and monomer-dimer interactions [15]. Equation 3 accurately predicts the observed fluorescence as a function of mole fraction, and therefore was used to predict changes in fluorescence in the donor and acceptor vesicles during the exchange process as described below. Inner filtering of light at the excitation and emission wavelengths was not responsible for the decreased fluorescence at higher mole fractions since both the fluorescence and absorbance increased linearly with concentration as the mole fraction was held constant (data not shown). The mole fraction of NBD-PC required for quantifiable self-quenching was much higher than that of any of the Bodipy-PC lipids.
Figure 1. Structures of phosphatidylcholine analogs used in this study.
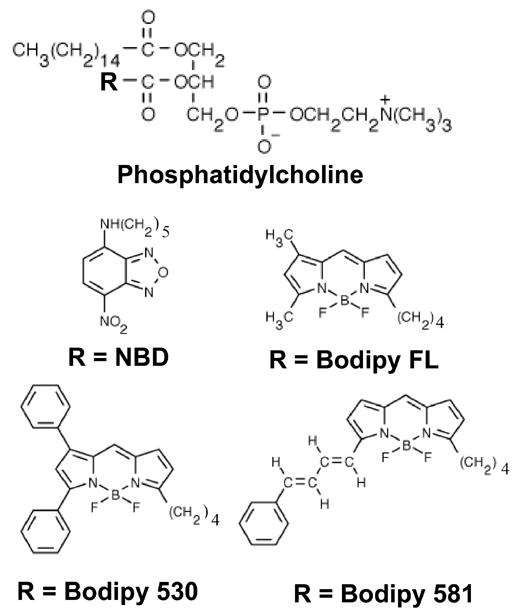
All fluorophores were attached to the core phosphatidylcholine molecule at the R position shown.
The rate of intervesicular transfer of fluorescent-PC analogues is highly dependent on the structure of the fluorophore
To measure the rate of spontaneous transfer between phospholipid vesicles, donor vesicles containing quenching concentrations of each of the fluorescent PC analogues were mixed with unlabeled acceptor vesicles, and the fluorescence increase was measured as the analogues transferred into the acceptor vesicles. Representative traces for each of the PC analogues are presented in Figure 3. The quenching curves predicted from the data in Figure 2 were used to relate the observed changes in fluorescence to the mass movement of the PC analogues between the vesicles. For a given donor and acceptor concentration, the equilibration of the analogues is a first order process regardless of the mechanism of transfer. Thus, the apparent first order rate constant was determined by regression analysis of the fluorescence traces using the quenching curves obtained from the data in Figure 2. The change in donor and acceptor vesicle fluorescence was predicted as the mole fraction in the donors decreased and the mole fraction in the acceptors increased. The predicted fluorescence for both the donors and acceptors is plotted in Figure 3. The apparent first order rate constants for each of the PC analogues are presented in Table 1. They differ by over four orders of magnitude depending on the structure of the fluorophore, ranging from 0.115 s−1 for NBD-PC to 5.9 × 10−6 s−1 for Bodipy 581-PC.
Figure 3. Representative intervesicular transfer traces.
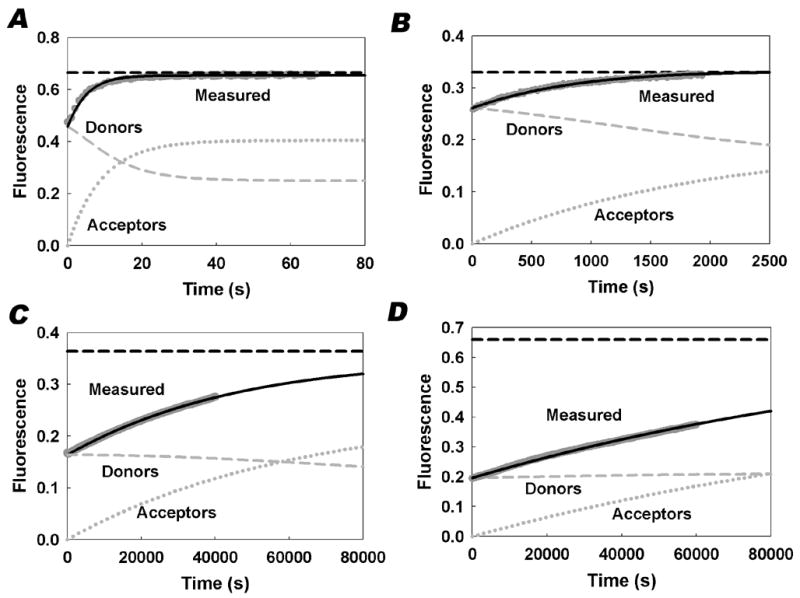
Transfer of each fluorescent PC analogue from donor vesicles to acceptor vesicles was monitored as an increase in fluorescence as described in “Materials and Methods.” Graphs are representative traces of NBD-PC (A), Bodipy FL-PC (B), Bodipy 530-PC (C), and Bodipy 581-PC (D). Experimental data are shown in dark gray. Equations 5–7 were used to generate the plots for the predicted total fluorescence (solid black line), the predicted fluorescence of the donor vesicles (gray dashed), and the predicted fluorescence of the acceptor vesicles (gray dotted). The black horizontal dashed line represents the predicted maximum fluorescence after equilibration.
Table 1. Summary of transfer rate measurements.
Values for the dissociation constant (k) and halftime to equilibrium (t½) were calculated as described in the Theoretical Basis section and represent the average ± the standard deviation of at least 3 independent transfer experiments.
| Fluorescent-PC | k (s−1) | t½ (s) | t½ relative to NBD-PC |
|---|---|---|---|
| NBD-PC | 1.15 × 10−1 ± 8.3 × 10−3 | 6.07 ± 0.43 | 1 |
| Bodipy FL-PC | 5.20 × 10−4 ± 2.58 × 10−6 | 1.33 × 103 ± 6.62 | 219 |
| Bodipy 530-PC | 1.52 × 10−5 ± 1.32 × 10−6 | 4.59 × 104 ± 3.96 × 103 | 7560 |
| Bodipy 581-PC | 5.90 × 10−6 ± 6.91 × 10−7 | 1.19 × 105 ± 1.48 × 104 | 19600 |
In previous studies, kinetic analysis of the intervesicular transfer of NBD-PC [16] and Bodipy FL-PC [11] demonstrated that the predominant mechanism of exchange was by the transfer of monomers through the water phase as opposed to the transfer resulting from transient vesicle collisions. To distinguish between these mechanisms for Bodipy 530-PC and Bodipy 581-PC, the apparent rate constant was measured as a function of the concentration of donor and acceptor vesicles. Increasing the concentration of donor and acceptor vesicles, while holding their ratio constant, had no effect on the measured apparent rate constant. This result rules out a vesicle collision-dependent mechanism, and we therefore concluded that the mechanism of spontaneous transfer for all four of the fluorescent PC analogues was by diffusion through the water phase. The theoretical description of this mechanism argues that the rate limiting step for this process is the dissociation of monomers from the vesicles. Thus the apparent first order rate constant can be interpreted to be the dissociation rate constant for monomeric dissociation from the donor and acceptor vesicles. The dissociation rate constant for a given amphiphile has been shown to correlate directly with its water solubility. The dissociation rate constant has been shown to decrease as a logarithmic function of the number of carbons in the acyl chains of fatty acids [18–20], lysophospholipids [21], and diacylphospholipids [10, 22]. Based on this correlation, one would predict that all of the Bodipy labeled PC analogues are significantly less water soluble than NBD-PC with Bodipy 581-PC being the most hydrophobic. However, since it has been shown recently that the association rate constant for diacyl phospholipid analogues [23] is several orders of magnitude slower than for their monoacyl counterparts [24], independent measurement of either the association rate constant or the partition coefficient is necessary to establish unambiguously the relative water solubility of these phospholipid analogues. Regardless of the magnitude of their water solubility, these data demonstrate a very large difference in the rate at which these phospholipid analogues gain access to the water phase from a bilayer membrane.
Although the flip-flop rates of NBD-PC [16] and Bodipy FL-PC [11] have been shown to be very slow relative to their rates of intervesicular transfer, given the slow rate of transfer of the Bodipy 530-PC and Bodipy 581-PC analogues, it was of interest to determine the extent to which flip-flop of these molecules from the inner to outer leaflet of the donor vesicles contributed to the amount of lipid transferred to the acceptors. Transfer experiments for these analogues were allowed to run for up to 15 hours. Analysis of the data from these longer runs indicated that the theoretical fit to the data was improved by the inclusion of a second exponential term presumably to account for the slower rate of flip-flop from the inner leaflet. The second exponential term for Bodipy 530-PC was 9.8 × 10−7 s−1 and for Bodipy 581-PC was 4.2 × 10−6 s−1 suggesting that the half-times for flip-flop were approximately 45 h and 200 h, respectively. The flip-flop rate for Bodipy FL-PC was previously measured to be 7.5 h [11]. These data reveal a direct correlation between the rate of intervesicular transfer and rate of flipflop which is consistent with that observed by Homan and Pownall comparing a homologous series of acyl chain labeled pyrene phosphatidylcholine analogues [25]. Although the addition of a second exponential term to account for a slow rate of flip-flop produces a better fit, the magnitude of the error would be small relative to the large differences observed between the different fluorescent labeled PC molecules.
DISCUSSION
The impetus for these studies was the observation that the mechanism of transport of PC analogues across the plasma membrane of yeast depends on the structure of the fluorescent label [5]. The deletion of the LEM3 gene alone and the DNF1 and DNF2 genes in combination almost completely blocks the inward-directed, transmembrane transport (flip) of NBD-PC, partially blocks that of Bodipy FL-PC, but has no effect on that of Bodipy 530-PC and Bodipy 581-PC. The measurements of the dissociation rate constants for these analogues presented here provide insight into the physical-chemical properties of these analogues that may explain their recognition by different transport pathways. The very large differences in dissociation rate constants, which are correlated with hydrophobicity, suggest that hydrophobicity may, at least partially, determine access of these PC analogues to particular transporters. For example, since NBD-PC rapidly dissociates from membranes, it could gain access to a binding site on a membrane transporter that is accessed only from the water. Thus, access to this transporter would depend on a fast dissociation rate constant, and it would be unlikely to transport Bodipy 530-PC and Bodipy 581-PC which have very slow dissociation rate constants. The endogenous substrate for this type of transporter would likely be the more water soluble degradation products of PC, lyso-PC or glycerophosphocholine. On the other hand, the more hydrophobic PC analogues, Bodipy 530-PC and Bodipy 581-PC might require transporters in which access to the binding site is by lateral diffusion in the plane of the membrane. Given that the half-times for spontaneous intervesicular transfer measured for Bodipy 530-PC (t½ = 12.8 h) and for Bodipy 581-PC (t½ = 33 h) more closely resemble that measured for the naturally occurring dimyristoyl-PC (t½ = 9.6 h [26]), these more hydrophobic PC analogues may better reflect the behavior of endogenous diacyl PC’s.
Acknowledgments
The authors thank Lynn Malone for her expert technical assistance. This work was supported by a National Institutes of Health grant GM064770 (to J.W.N.) and a grant from the University Research Committee of Emory University.
The abbreviations used are
- Bodipy FL-PC
2-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine
- Bodipy 530-PC
2-(4,4-difluoro-5,7-diphenyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine
- Bodipy 581-PC
2-(4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine
- HBS
HEPES buffered saline
- NBD
7-nitrobenz-2-oxa-1,3-diazol-4-yl
- NBD-PC
1-myristoyl-2-[6-(NBD) aminocaproyl]-phosphatidylcholine
- PC
phosphatidylcholine
- POPC
1-palmitoyl, 2-oleoyl phosphatidylcholine
Footnotes
This work was supported by NIH grant GM064770 (to J.W.N.) and a grant from the University Research Committee of Emory University (to J.W.N.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heikinheimo L, Somerharju P. Translocation of pyrene-labeled phosphatidylserine from the plasma membrane to mitochondria diminishes systematically with molecular hydrophobicity: implications on the maintenance of high phosphatidylserine content in the inner leaflet of the plasma membrane. Biochim Biophys Acta. 2002;1591:75–85. doi: 10.1016/s0167-4889(02)00253-7. [DOI] [PubMed] [Google Scholar]
- 2.Nichols JW. Internalization and trafficking of fluorescent-labeled phospholipids in yeast. Semin Cell Dev Biol. 2002;13:179–184. doi: 10.1016/s1084-9521(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 3.Pomorski T, Holthuis JC, Herrmann A, van Meer G. Tracking down lipid flippases and their biological functions. J Cell Sci. 2004;117:805–813. doi: 10.1242/jcs.01055. [DOI] [PubMed] [Google Scholar]
- 4.Marks DL, Singh RD, Choudhury A, Wheatley CL, Pagano RE. Use of fluorescent sphingolipid analogs to study lipid transport along the endocytic pathway. Methods. 2005;36:186–195. doi: 10.1016/j.ymeth.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Elvington SM, Bu F, Nichols JW. Fluorescent, acyl chain-labeled phosphatidylcholine analogs reveal novel transport pathways across the plasma membrane of yeast. J Biol Chem. 2005;280:40957–40964. doi: 10.1074/jbc.M507926200. [DOI] [PubMed] [Google Scholar]
- 6.Chattopadhyay A, London E. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry. 1987;26:39–45. doi: 10.1021/bi00375a006. [DOI] [PubMed] [Google Scholar]
- 7.Wolf DE, Winiski AP, Ting AE, Bocian KM, Pagano RE. Determination of the transbilayer distribution of fluorescent lipid analogues by nonradiative fluorescence resonance energy transfer. Biochemistry. 1992;31:2865–2873. doi: 10.1021/bi00126a004. [DOI] [PubMed] [Google Scholar]
- 8.Huster D, Muller P, Arnold K, Herrmann A. Dynamics of membrane penetration of the fluorescent 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD) group attached to an acyl chain of phosphatidylcholine. Biophys J. 2001;80:822–831. doi: 10.1016/S0006-3495(01)76061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser RD, London E. Determination of the depth of BODIPY probes in model membranes by parallax analysis of fluorescence quenching. Biochim Biophys Acta. 1998;1375:13–22. doi: 10.1016/s0005-2736(98)00127-8. [DOI] [PubMed] [Google Scholar]
- 10.Nichols JW. Thermodynamics and kinetics of phospholipid monomer-vesicle interaction. Biochemistry. 1985;24:6390–6398. doi: 10.1021/bi00344a011. [DOI] [PubMed] [Google Scholar]
- 11.Bai J, Pagano RE. Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry. 1997;36:8840–8848. doi: 10.1021/bi970145r. [DOI] [PubMed] [Google Scholar]
- 12.Hope MJ, Bally MB, Webb G, Cullis PR. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim Biophys Acta. 1985;812:55–65. doi: 10.1016/0005-2736(85)90521-8. [DOI] [PubMed] [Google Scholar]
- 13.Hagihara B, Lardy HA. A method for the separation of orthophosphate from other phosphate compounds. J Biol Chem. 1960;235:889–894. [PubMed] [Google Scholar]
- 14.MacDonald RI. Characteristics of self-quenching of the fluorescence of lipid-conjugated rhodamine in membranes. J Biol Chem. 1990;265:13533–13539. [PubMed] [Google Scholar]
- 15.Arbeloa IL. Dimeric and trimeric states of the fluorescein dianion. J Chem Soc Faraday Trans 2. 1981;77:1735–1742. [Google Scholar]
- 16.Nichols JW, Pagano RE. Use of resonance energy transfer to study the kinetics of amphiphile transfer between vesicles. Biochemistry. 1982;21:1720–1726. doi: 10.1021/bi00537a003. [DOI] [PubMed] [Google Scholar]
- 17.Nichols JW, Pagano RE. Kinetics of soluble lipid monomer diffusion between vesicles. Biochemistry. 1981;20:2783–2789. doi: 10.1021/bi00513a012. [DOI] [PubMed] [Google Scholar]
- 18.Doody MC, Pownall HJ, Kao YJ, Smith LC. Mechanism and kinetics of transfer of a fluorescent fatty acid between single-walled phosphatidylcholine vesicles. Biochemistry. 1980;19:108–16. doi: 10.1021/bi00542a017. [DOI] [PubMed] [Google Scholar]
- 19.Zhang F, Kamp F, Hamilton JA. Dissociation of long and very long chain fatty acids from phospholipid bilayers. Biochemistry. 1996;35:16055–60. doi: 10.1021/bi961685b. [DOI] [PubMed] [Google Scholar]
- 20.Kamp F, Hamilton JA. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot Essent Fatty Acids. 2006;75:149–59. doi: 10.1016/j.plefa.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Nichols JW. Protein-mediated transfer of fluorescent-labeled phospholipids across brush border of rabbit intestine. Am J Physiol. 1994;267:G80–6. doi: 10.1152/ajpgi.1994.267.1.G80. [DOI] [PubMed] [Google Scholar]
- 22.Massey JB, Gotto AM, Jr, Pownall HJ. Kinetics and mechanism of the spontaneous transfer of fluorescent phosphatidylcholines between apolipoprotein-phospholipid recombinants. Biochemistry. 1982;21:3630–6. doi: 10.1021/bi00258a016. [DOI] [PubMed] [Google Scholar]
- 23.Abreu MS, Moreno MJ, Vaz WL. Kinetics and thermodynamics of association of a phospholipid derivative with lipid bilayers in liquid-disordered and liquid-ordered phases. Biophys J. 2004;87:353–65. doi: 10.1529/biophysj.104.040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sampaio JL, Moreno MJ, Vaz WL. Kinetics and thermodynamics of association of a fluorescent lysophospholipid derivative with lipid bilayers in liquid-ordered and liquid-disordered phases. Biophys J. 2005;88:4064–71. doi: 10.1529/biophysj.104.054007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Homan R, Pownall HJ. Transbilayer diffusion of phospholipids: dependence on headgroup structure and acyl chain length. Biochim Biophys Acta. 1988;938:155–66. doi: 10.1016/0005-2736(88)90155-1. [DOI] [PubMed] [Google Scholar]
- 26.Wimley WC, Thompson TE. Exchange and flip-flop of dimyristoylphosphatidylcholine in liquid-crystalline, gel, and two-component, two-phase large unilamellar vesicles. Biochemistry. 1990;29:1296–1303. doi: 10.1021/bi00457a027. [DOI] [PubMed] [Google Scholar]


