Abstract
Juvenile hormone esterase (JHE) plays an important role in regulating juvenile hormone titers. Recent sequencing and annotation of the Aedes aegypti genome identified ten putative jhe gene sequences. Analysis of these ten putative jhe gene sequences showed that only three of them, EAT43357, EAT43353 and EAT43354 contained GQSAG motif and showed high sequence similarity with the sequences of jhe genes identified from other insect species. To determine which putative jhe gene(s) code for functional JHE, the mRNA profiles of EAT43357, EAT43353 and EAT43354 were measured during the final instar larval and pupal stages by using quantitative real-time reverse transcriptase PCR. The mRNA for EAT43357 was detected during the late final instar larval stage. In contrast, EAT43354 mRNA was detected only during the pupal stage and EAT43353 mRNA was detected only during the larval stage. The mRNA of EAT43357 was detected in both fat body and midgut tissues. JHE enzyme levels gradually increased during the final instar larval stage reaching a peak at 42 hr after ecdysis into the final instar larval stage. The mRNA expression profiles of EAT43357 correlate with the developmental expression profiles of JHE enzyme activity suggesting that this gene may encode for a functional JHE. The EAT43357 and EAT43354 cDNA were expressed in a baculovirus system. Proteins isolated from Sf9 cells infected with recombinant baculovirus expressing EAT43357 but not EAT43354 gene exhibited JHE activity confirming that EAT43357 gene codes for a functional JHE enzyme.
Keywords: Insect, hormones, baculovirus expression, gene identification, mRNA profiles, development
1. Introduction
I (S.R.P) was introduced to insect endocrinology when I had an opportunity to train in Professor Riddiford’s laboratory from 1988–1992. Professor Riddiford made numerous contributions towards understanding of both biological and molecular action of insect hormones. She was involved in pioneering work on JH and commitment, and worked out 20-hydroxyecdysone (20E) and juvenile hormone (JH) regulation of gene expression during molting and metamorphosis in Manduca sexta. The titers of 20E and JH and their effect on insect development have been one of the focal points of Professor Riddiford’s research. Juvenile hormone esterase (JHE) plays a critical role in controlling titers of JH during insect development. We are honored to dedicate this paper describing identification of JHE from mosquito, Aedes aegypti to Professor Riddiford on the occasion of her 70th birthday.
The sesquiterpenoid, JH and ecdysteroid, 20E are among the hormones that regulate insect metamorphosis and development. JH acts as a status quo hormone and modulates 20E action to prevent metamorphosis (Riddiford 1996). During the larval-pupal metamorphosis, JH is removed from the hemolymph and tissues at precise times by controlling JH biosynthesis as well as JH degradation. Two JH-specific metabolizing enzymes, JH esterase (JHE) and JH epoxide hydrolase (JHEH), are mainly responsible for the degradation of JH. JHE metabolizes JH by hydrolyzing the α/β unsaturated methyl ester to the JH acid, while JHEH hydrates the epoxide ring resulting in JH diol (Hammock 1985).
Because of its critical role in regulating JH titers during insect development, JHE has been a target during the development of biorational insecticides. Application of a chemical JHE inhibitor containing a trifluoromethylketone (TFK), such as 3-octylthio-1,1,1-trifluoro-2-propanone (OTFP) delayed the pupation of lepidopteran larvae (Hammock et al. 1984). Recombinant Autographa californica multiple nucleopolyhedro virus (AcMNPV) expressing a lepidopteran jhe gene caused a reduction in feeding of final instar larvae (Hammock et al. 1990; Bonning et al. 1995). Infection with a recombinant baculovirus constructed with the jhe gene from Heliothis virescens in the antisense orientation greatly reduced the hemolymph JHE level and resulted in abnormal metamorphosis of the final instar of H. virescens larvae (Hajos et al. 1999).
JHEs have been identified in many insect species. JHE proteins have been purified from Heliothis virescens (Hanzlik et al. 1989), Leptinotarsa decemlineata (Vermunt et al. 1997b), Manduca sexta (Venkatesh et al. 1990), Trichoplusia ni (Hanzlik and Hammock 1987), Drosophila melanogaster (Campbell et al., 1998), Tenebrio moliter (Thomas et al. 2000) and Bombyx mori (Shiotsuki et al. 2000). JHE cDNAs have been cloned from several insect species such as H. virescens (Hanzlik et al. 1989), L. decemlineata (Vermunt et al. 1997a), Choristoneura fumiferana (Feng et al. 1999) and B. mori (Hirai et al. 2002). To further understand the interaction between JHE and its substrates and inhibitors, the crystal structure of the JHE protein from Manduca sexta was solved. These studies showed that JHE contains a long hydrophobic binding pocket with solvent-inaccessible catalytic triad and OTFP can covalently bind to the catalytic serine site (Wogulis et al. 2006).
Genome sequencing and annotation can help to identify new genes without using traditional cDNA cloning methods. Recent sequencing and annotation of the Aedes aegypti genome identified 10 sequences that may code for JHE. In this study, we selected three Ae. aegypti putative jhe genes that contained most of the functional JHE motifs and investigated their expression during the final instar larval and pupal stages. We also determined JHE enzyme titers during the final instar larval and pupal stages. These studies suggested that one of the three genes, EAT43357, may code for JHE enzyme. EAT43357 gene was expressed in a baculovirus protein expression system and the proteins isolated from virus infected cells exhibited JHE activity confirming that EAT43357 gene encodes a functional JHE enzyme.
2. Materials and methods
2.1 Mosquito rearing and staging
Aedes aegypti larvae and pupae were reared at 27 ± 1 °C with a light:dark cycle of 16:8. Eggs of Ae. aegypti were hatched synchronously in a vacuum chamber for 15 min (Christophers 1960). Larvae were fed on bovine liver powder solution (MP Biochemicals, LLC, Aurora, OH) and the density of 3rd and 4th instar larvae was maintained at one larvae per milliliter. Under these rearing conditions, larval and pupal stages were completed in 7 days. The un-tanned head (white head) character was used to identify newly molted larvae and pupae, and the developing pupal respiratory trumpets were used to idenitfy pharate pupa (Nishiura 2002; Wu et al., 2006).
2.2 RNA isolation and cDNA synthesis
At each time point, five larvae or pupae were pooled and homogenized in TRI reagent (Molecular Research Center Inc., Cincinnati, OH). Midgut tissues from staged larvae and pupae were dissected in phosphate buffered saline (PBS) treated with 0.1% diethylpyrocarbonate (DEPC). About 15 larval and 30 pupal midguts were pooled and homogenized in TRI reagent. Total RNA of whole body and midgut were isolated according to the manufacturer’s instructions. Total RNA was treated with DNase I (Ambion, Austin, TX) in a 20 μl total reaction volume following the manufacturer’s protocol. cDNA synthesis by reverse transcription was performed using 2 μg of DNase I treated RNA and iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) in a 20 μl reaction volume.
2.3 Quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR)
Developmental changes in the mRNA levels were measured using qRT-PCR using a MyiQ single color real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA). qRT-PCR was performed in a 20 μl total reaction volume containing 1 μl of cDNA, 1 μl primer mix containing 10 mM each of forward and reverse gene specific primers (Table 1A), 8 μl of H2O and 10 μl of SYBER Green supermix (Bio-Rad Laboratories, Hercules, CA). The qRT-PCR was done with the following the cycling regime: initial incubation of 95 °C for 3 min; 45 cycles of 95°C for 10 s, 60 °C for 20 s, 72 °C for 30 s; and a final extension of 72 °C for 8 min. Standard curves were obtained using a ten-fold serial dilution of pooled midgut cDNAs from all of the stages. Ribosomal protein subunit S7RP was used as an internal control. The mRNA expression from each gene was quantified in relation to the expression of S7RP. The primer pair of each gene was designed to amplify about 200 bp PCR products. Only data that showed good efficiency (≥85%) and correlation coefficient (≥0.95) were included in the analysis. Means and Standard errors for each time point were obtained from the average of three independent sample sets.
Table 1A.
Primers used in RT-PCR
2.4 In situ hybridization
In situ hybridization was performed to investigate the tissue specific localization of JHE mRNA. The PCR product (173 bp) of EAT43357 was cloned into the pGEM-T vector (Promega Corporation, Madison, WI) and the RNA probe was synthesized by using a T7/SP6 DIG RNA labeling kit (Roche Diagnostics Corporation, Indianapolis, IN). The midguts and epidermis with fat bodies were dissected in 1X PBS (phosphate-buffered saline) and fixed in 4% paraformaldehyde overnight at 4 °C. The tissues were dehydrated with a graded series of methanol:PBS. The tissues were permeabilized in 100% methanol for 20 min and rehydrated with a graded series of PBT (1× PBS +0.1% Tween20): methanol. Protein digestion was performed by incubating with proteinase K (10μg/ml) for 15 min at 37° C. The tissues were incubated with the RNA probes (both sense and antisense) in hybridization mix (50% formamide + 5X SSC + salmon sperm DNA 0.1 mg/ml, tRNA 0.1 mg/ml, heparin 0.05 mg/ml) at a concentration of 5–10 ng/ml. Hybridization reactions were carried out at 55 °C overnight with 100 ml of hybridization mix. After hybridization, the tissues were washed three times, 10 min each with hybridization mix without probes followed by two washes for 10 min each with 1:1 hybridization mix:PBT and finally washed with PBT alone. The tissues were then blocked with 1% normal goat serum for 1 hr at room temperature and incubated with anti-DIG conjugated with rhodamine (Roche, 1:1000 dilution) overnight at 4° C. The tissues were washed extensively with PBT for 2 hr, counterstained with DAPI (10 μg/ml) and mounted in 50% glycerol. The tissues were visualized using a Leica TCS-SP laser scanning confocal microscope and documented using Leica TCS software.
2.5 Production of recombinant AaJHE
EAT43357, EAT43353 and EAT43354 recombinant proteins were produced using the BaculoDirect Baculovirus protein expression systems (Invitrogen, Carlsbad, CA). Primers containing attB recombination sites were designed to amplify the whole open reading frames of EAT43357, EAT43353 and EAT43354 (Table 1B). cDNA was synthesized from the RNA isolated from the final instar larvae using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). EAT43357, EAT43353 and EAT43354 cDNAs were amplified using the following cycling conditions: initial incubation of 94 °C for 2 min; 20 cycles of 94 °C for 30 s, 52 °C for 30 s, 72 °C for 2 min; 10 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 2 min; Final extension of 72 °C for 8 min. The amplified PCR products (flanked with attB site) were then used in a BP recombination reaction with pDONR 201 vector (containing attP site) (Invitrogen, Carlsbad, CA) to generate the entry clone called pENTR/AaJHE, following the methods described in the Gateway Cloning Technology manual (Invitrogen, Carlsbad, CA). The recombinant AaJHEs were produced through an LR recombination reaction between pENTR/AaJHE (containing attL site) and BaculoDirect Linear DNA (containing attR site) containing a C-terminal V5 epitope and 6XHis tag and the insertion was confirmed by PCR using the putative AaJHE specific forward primer and V5 reverse primer.
Table 1B.
Primers used for expression of proteins in baculovirus protein expression system. attB sites are underlined
Sf9 cells were cultured in Hink’s TNM-FH insect medium (SAFC Biosciences, Lenexa, KS) supplemented with 10% fetal bovine serum (FBS) at 27 °C. Transfections were performed by incubating 8×105 Sf9 cells per well in six-well plates with three putative jhe DNA constructs and cellfectin reagent (Invitrogen). At five days post transfection, medium was collected as P1 viral stock and stored at 4 °C. Sf9 cells were infected with 200μl P1 viral stock in T-25 tissue culture flasks and medium was collected as P2 viral stock three days post infection. Then at 2 days post transfection, the cells that infected with 500 μl P2 viral stock were harvested and centrifuged at 800x g for 5 min at 4°C. Cell pellets were washed once with 1x PBS and resuspended in 250μl lysis buffer (10 mM Na2HPO4, 30 mM NaCl, 0.25% Tween 20, 10 mM EDTA, 10 mM EGTA, pH 7.0) with 0.1% proteinase inhibitor cocktail (Sigma, St. Louis, MO) and 0.1 mM Phenylmethylsulphonylfluoride (PMSF). Resuspended cells were sonicated for 15 sec three times using Tekmar sonic disruptor (Tekmar Co., Cincinnati, OH) and centrifuged at 14,000x g for 15 min at 4°C. Protein concentrations were estimated by Bradford assay (Bradford 1976) and the supernatants were used for both western blotting and JHE activity assay.
2.6 Western blotting
Thirty μg of protein samples were denatured at 95°C for 5 min in 1x loading buffer (50 mM Tris-Cl, pH 6.8, 100 mM dithiothreitol, 2% sodium dodecyl sulfate [SDS], 0.1% bromophenol blue, 10% glycerol). Denatured protein samples were loaded and separated on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) using transfer buffer (39 mM glycine, 48 mM Tris, 0.037% SDS, 20% MeOH, pH 8.3) and a Trans-blot semidry transfer cell (Bio-Rad). Membranes were blocked in 5% blotto solution (BLOT-Quickblocker; GENO Technology, Inc. St. Louis, MO) in TTBS buffer (100 mM Tris-HCl, 0.9% NaCl, 0.1% Tween 20, pH 7.5) for 1 hr, and incubated with 1:5000 anti-V5 antibody produced in rabbit (Sigma, St. Louis, MO) overnight at 4°C. Membranes were washed in TTBS and incubated with 1:5000 goat anti-rabbit IgG conjugate with alkaline phosphatase (Sigma, St. Louis, MO) for 1 hr. After washing with TTBS and alkaline phosphatase substrate buffer (0.1 M NaCl, 5 mM MgCl2, 100 mM Tris-HCl, pH 9.5), recombinant proteins were visualized by exposing the membrane to the mixture of nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Sigma, St. Louis, MO).
2.7 JHE activity assay
Juvenile hormone III was purchased from Sigma (St. Louis, MO). [10-3H(N)]-JH III (17.5 Ci/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA). OTFP was kindly provided by Dr. Bruce D. Hammock, University of California-Davis.
The enzymatic activity of recombinant AaJHEs and whole body samples were measured using the partition assay described previously (Hammock and Sparks 1977; Share and Roe 1988). Briefly, 100 μl aliquot of cell lysates or whole body homogenates (about 1 mg crude protein) in 1X PBS were incubated with 1 μl 100% ethanol or 1 μl 0.002 M OTFP for 10 min at 30 °C, followed by incubation with [3H]-labeled (130,000 DPM) JHIII and unlabeled JH III (final JH III concentration was 5 × 10−4 M) for 30 min at 30 °C. The reaction was stopped by adding 50 μl of methanol:water:ammonium hydroxide solution (10:9:1, v/v/v). The solution was extracted with 250 μl isooctane. The lower phase (aqueous) and the upper phase (organic) were counted for radioactivity to estimate the degradation of JH III. The JHE enzymatic activity was determined by subtracting non-specific activity (OTFP group) from total activity (ethanol group).
3. Results
3.1 Identification of putative jhe genes
Recently, ten sequences designated as juvenile hormone esterase of Aedes aegypti were submitted to NCBI database by Broad Institute and the Institute for Genomic Research. Alignment of deduced amino acid sequence from these ten genes showed that not all the putative jhe genes contain conserved catalytic motifs that are required for JHE function (Fig. 1). Most of the putative jhe genes contained RF, DQ and GxxHxxD motifs. Only three of these genes, EAT43353, EAT43354 and EAT43357 contained GQSAG motif, the core catalytic motif required for JH-specific esterase activity (Table 2). In addition, EAT43354 and EAT43357 sequences contained aspartic acid in place of glutamic acid in the E motif. Drosophila melanogaster JHE (DmJHE) contains glutamic acid at this position. Interestingly, these three putative jhe genes (EAT43353, EAT43354 and EAT43357) are located in the same SuperContig (supercont1.145) of the Ae. aegypti genome (Accession # AAGE02008108.1). The amino acid sequence of EAT43357 showed the highest (41.9%) similarity with the amino acid sequence of DmJHE. Since partial sequence of EAT43353 was reported, we used the GENSCAN program (http://genome.dkfz-heidelberg.de/cig-bin/GENSCAN/genscan.cgi) to predict the full length ORF (547 aa) of this gene.
Fig. 1.
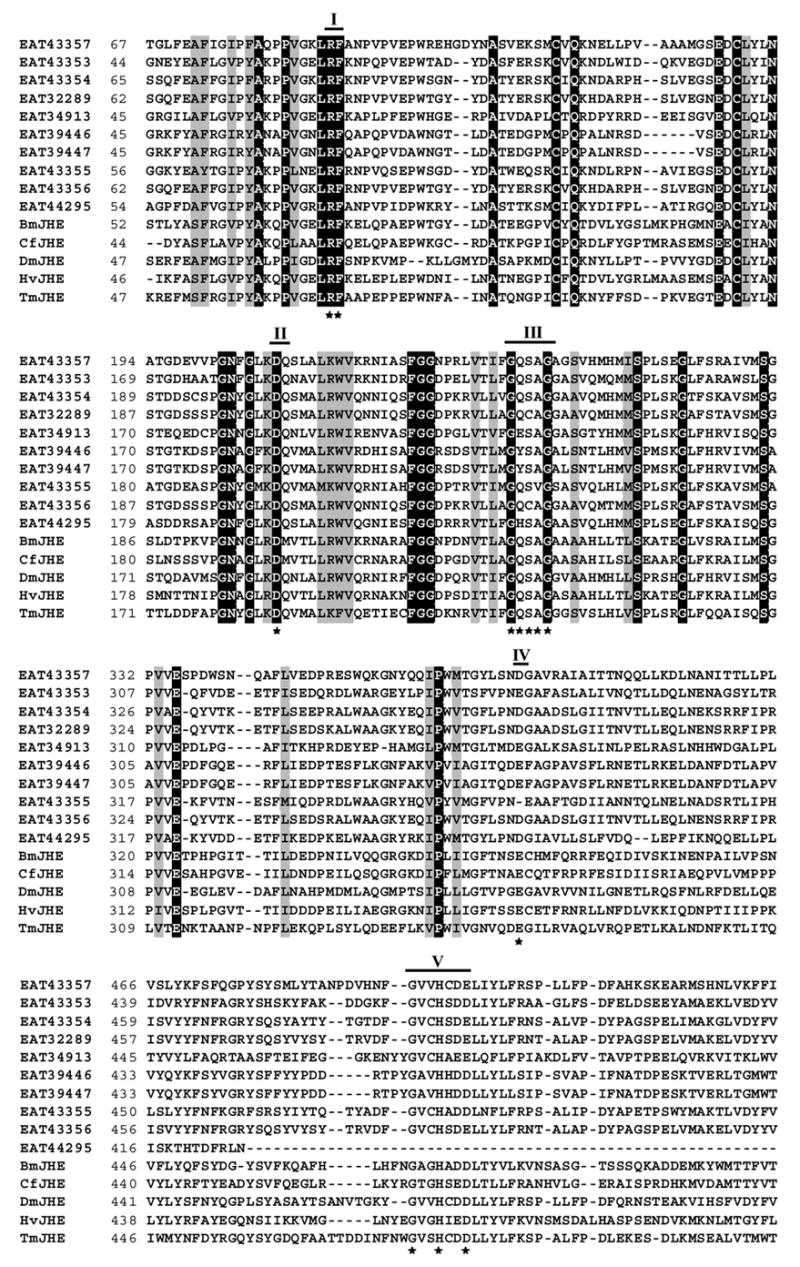
Alignment of amino acid sequences of ten putative jhe genes from Aedes aegypti, and jhe genes of Drosophila melanogaster (DmJHE, Accession # NM_079034), Choristoneura fumiferana (CfJHE, Accession # AAD34172), Tenebrio molitor (TmJHE, Accession # AAL41023), Heliothis virescens (HvJHE, Accession # AAC38822), Bombyx mori (BmJHE, Accession # AAR37335), using ClustalW program (http://www.ebi.ac.uk/clustalw) and BioEdit Sequence Alignment Editor (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Five conserved catalytic motifs are indicated by bold lines with the numbers on the top. Numbers on the left show the position of amino acids in each protein. Letters with black and gray shading denote perfect and similar identities, respectively. The motifs important for JHE function are shown as stars. GENSCAN program (http://genome.dkfz-heidelberg.de/cig-bin/GENSCAN/genscan.cgi) was used to predict the full length ORF (547 aa) of EAT43353 gene
Table 2.
Sequence analysis of ten putative JHEs from Aedes aegypti. Three putative AaJHEs that contain the GQSAG motif are shaded.
| RF motif | DQ motif | GQSAG motif | E motif | GxxHxxD/E motif | Identity to DmJHE | |
|---|---|---|---|---|---|---|
| EAT43357 | Yes | Yes | Yes | No | Yes | 41.9% |
| EAT43354 | Yes | Yes | Yes | No | Yes | 35.3% |
| EAT43353 | Yes | Yes | Yes | Yes | Yes | 35.2% |
| EAT43355 | Yes | Yes | No | Yes | Yes | 34.0% |
| EAT32289 | Yes | Yes | No | No | Yes | 32.6% |
| EAT43356 | Yes | Yes | No | No | Yes | 32.1% |
| EAT34913 | Yes | Yes | No | Yes | Yes | 31.2% |
| EAT44295 | Yes | Yes | No | No | No | 29.3% |
| EAT39446 | Yes | Yes | No | Yes | Yes | 28.2% |
| EAT39447 | Yes | Yes | No | Yes | Yes | 26.8% |
We performed signal peptide scan on the three putative jhe gene sequences using SignalP 3.0 program (Technical University of Denmark, http://www.cbs.dtu.dk/services/SignalP). The results showed that all three pupative jhe genes contain sequences coding for signal peptides. About 5 kb of the promoter region of the three putative jhe genes (EAT43353, EAT43354 and EAT43357) were submitted to the MSAT program (Motif Alignment and Search Tool, http://meme.sdsc.edu/meme/mast.html) to search for the presence of JH response elements (JHRE) identified previously (Kethidi et al., 2004). The alignment results showed that EAT43357 promoter contained a JHRE (RGRNYANNNNRGRNYA) located between −2729 and −2715.
3.2 Developmental expression of three putative Aajhe genes
Quantitative real-time RT-PCR was used to measure the mRNA expression profiles of the three putative jhe genes in both whole body homogenates and midgut. The three genes showed different expression profiles during the final instar larval and pupal stages.
In whole body homogenates, the EAT43357 mRNA levels were low at the beginning of the final instar larval stage and started to increase at 27 hr after ecdysis to the final instar larval stage (AEFL) and reached a maximum at 42 hr AEFL and then decreased and reached low levels prior to pupation (Fig. 2A). The levels of EAT43357 mRNA were low during the pupal stage. Both the expression levels and the developmental expression pattern of EAT43357 in the midgut during the final instar larval stage are similar to those observed in the whole body (Fig. 2A).
Fig. 2.
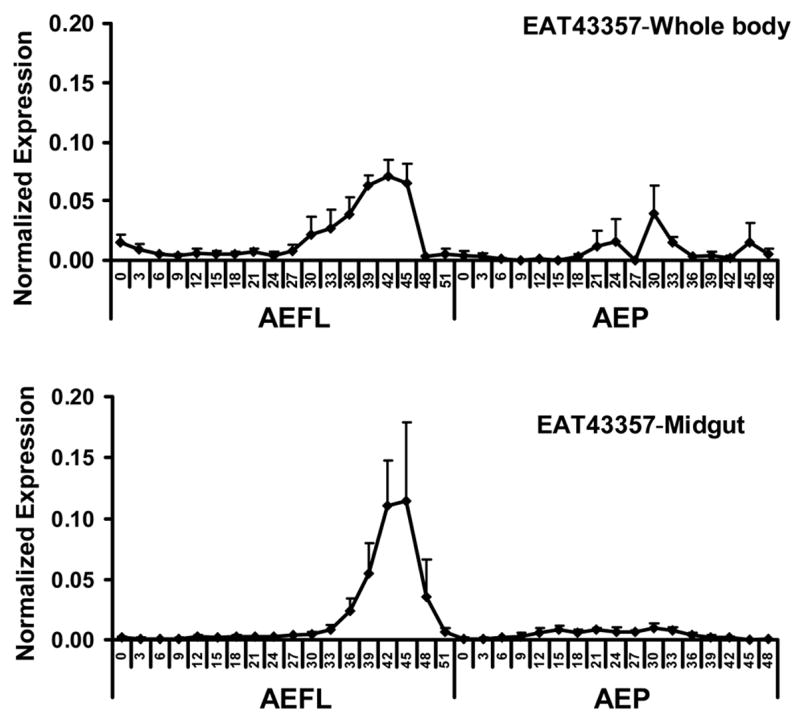
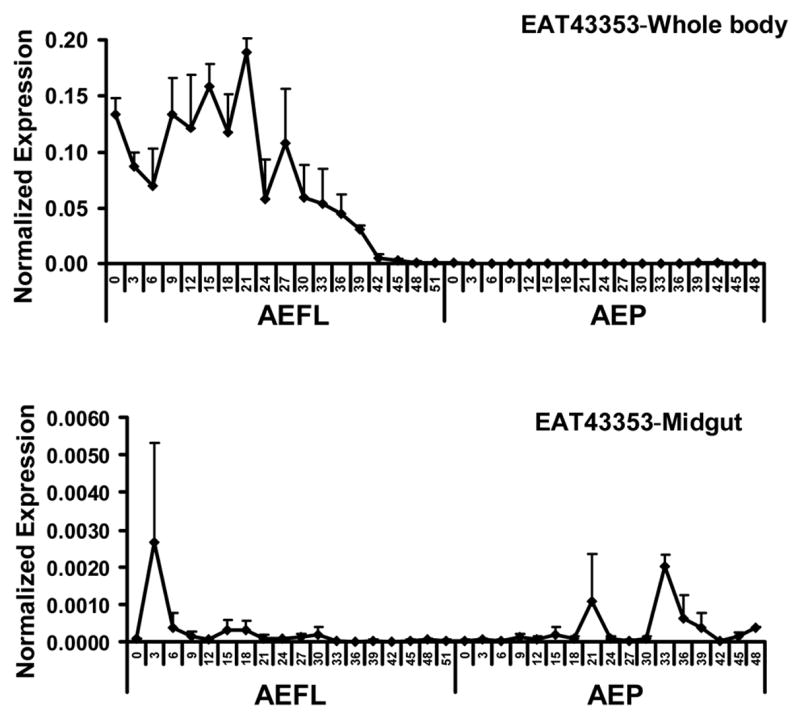
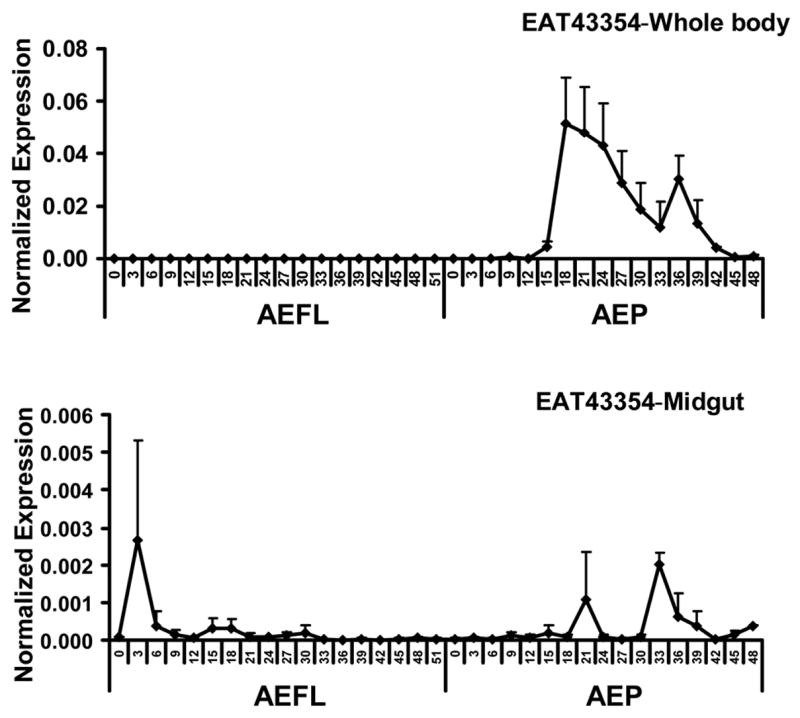
Developmental profiles of EAT43357 (A), EAT43353 (B) and EAT43354 (C) mRNA in whole body homogenates and midgut. Newly molted 4th instar larvae were sampled every 3 hr until pupation. Newly molted pupae were sampled every 3 hr until adult emergence. The pharate pupae with tanned respiratory trumpets appearing at 48 hr and 51hr AEFL were also staged. The mRNA levels of these genes were normalized using S7RP ribosomal RNA as an internal standard. Mean ± SE for three independently staged sets of larvae and pupae are shown. AEFL, after ecdysis into the final instar larval stage; AEP, after ecdysis into the pupa stage.
The EAT43353 mRNA levels of the whole body homogenates were higher at the early stages of the final instar larval stage (Fig. 2B). The mRNA reached a peak at 21 hr AEFL, then declined and reached undetectable levels by 48 hr AEFL. No EAT43353 mRNA was detected during the pupal stage. Only, low levels of EAT43353 mRNA were detected in the midgut (Fig. 2B).
Only low levels of EAT43354 mRNA were detected during the final instar larval stage. The EAT43354 mRNA levels in the whole body increased during the pupal stage and reached a peak between 18 hr and 33 hr after ecdysis to the pupal stage. Then the mRNA levels gradually decreased and reached low levels prior to adult emergence (Fig. 2C). Similar to EAT43353, low levels of EAT43354 mRNA were detected in the midgut.
3.3 Detection of EAT43357 mRNA in the fat body and midgut
In most insects, the fat body is the primary source of secreted JHE. As shown in Figure 2A, EAT43357 mRNA was detected in the midgut of Ae. aegypti. In situ hybridization was performed to further confirm the expression of EAT43357 in both the fat body and midgut. The tissues were dissected from the larvae that were at 45 hr AEFL because EAT43357 is highly expressed in both whole body homogenates and the midgut at this stage. EAT43357 mRNA was detected in the fat body cells, but not in the epidermal cells (Fig. 3A). EAT43357 mRNA was detected in both large larval and imaginal midgut cells (Fig. 3B). No signal was detected when sense RNA was used as a probe.
Fig. 3.
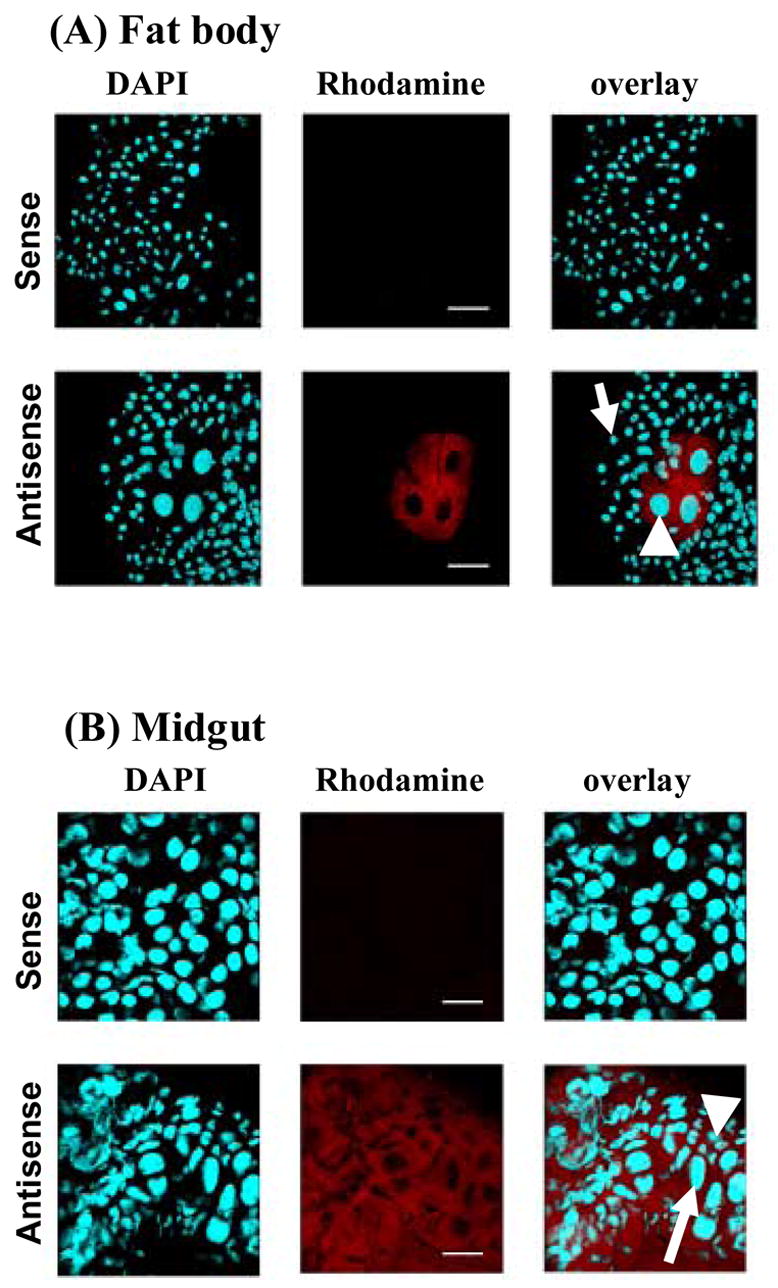
Tissue specific expression of EAT43357 at 45 hr AEFL in the fat body (A) and midgut (B). In situ hybridization was performed on whole mounts of fat body and midgut tissues using a DIG labeled antisense mRNA probe for EAT43357. Nuclear staining was performed with DAPI. The transcripts were localized using anti-DIG Rhodamine conjugate. Sense strand RNA probes were used as the negative control shown in the top panel of each tissue. No gene expression was detected in the negative control panels. (A). EAT43357 mRNA was detected in fat body cells (white arrowhead), but not in the epidermal cells (white arrow); (B). EAT43357 mRNA was detected in both large larval (white arrow) and small imaginal midgut cells (white arrowhead). Scale bar: 100 μm.
3.4 The JHE enzymatic activity correlates with the mRNA expression of EAT43357 gene during the final instar larval stage
The JHE enzymatic activity was measured by partition assay using JH III as a substrate. JHE activity was low at the beginning of the final instar larval stage. The JHE activity started to increase at 24 hr AEFL and reached the maximum levels at 42 hr AEFL, and the high level of JHE activity was maintained until pupation (Fig. 4). The developmental changes in JHE enzymatic activity during the final instar larval stage correlated well with the developmental expression pattern of EAT43357 mRNA suggesting that this gene may code for functional JHE. In contrast, the developmental changes in JHE enzymatic activity did not correlated well with the developmental expression pattern of EAT43353 or EAT43354 mRNAs.
Fig. 4.
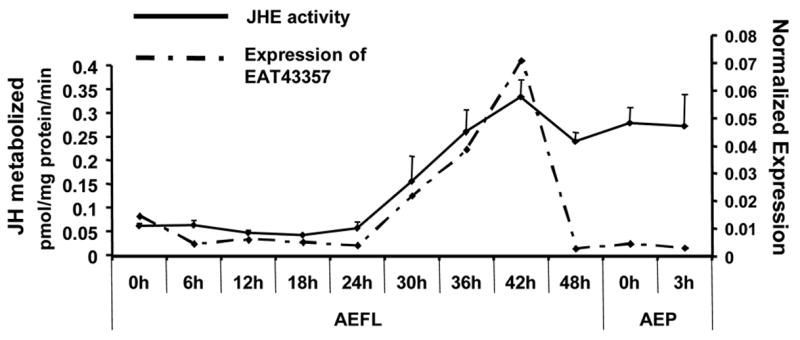
Developmental profile of JHE enzymatic activity. JHE activity (solid line) was determined in whole body extracts collected at 6 hr intervals during the final instar larval stage and early pupal stage. For each time point, five larvae or pupae were collected. Mean ± SE for three independently staged sets of larvae and pupae are shown. The mRNA expression profile of EAT43357 is shown as a dashed line.
3.5 Production of recombinant AaJHE protein in a baculovirus protein expression system
Three putative jhe genes (EAT43357, EAT43353 and EAT43354) were expressed using BaculoDirect Baculovirus protein expression systems (Invitrogen). Western blot analysis of proteins isolated from cells infected with AcMNPV expressing EAT43357 and EAT43354 genes showed bands at about 75 kDa. These observed molecular weights are closer to the predicted sizes, 71 kDa and 69 kDa for EAT43357 and EAT43354 proteins respectively (Fig. 5A). We could not detect recombinant EAT43353 protein using western blots. Proteins isolated from Sf9 cells infected with AcMNPV expressing EAT43357 and EAT43354 were used in JHE activity assays. The proteins isolated from cells expressing EAT43357 metabolized JH III (0.226 pmoles JH III/mg protein/min). When JHE inhibitor (OTFP) was added to the reaction mixture, the JHE activity was reduced ten-fold (0.019 pmoles JH III/mg protein/min) (Fig. 5B). No JHE enzymatic activity was detected in the proteins isolated from cells infected with wild-type AcMNPV or recombinant virus expressing EAT43354 gene. These data show that the EAT43357 gene codes for a functional JHE enzyme and we designated this gene as Aajhe.
Fig. 5.
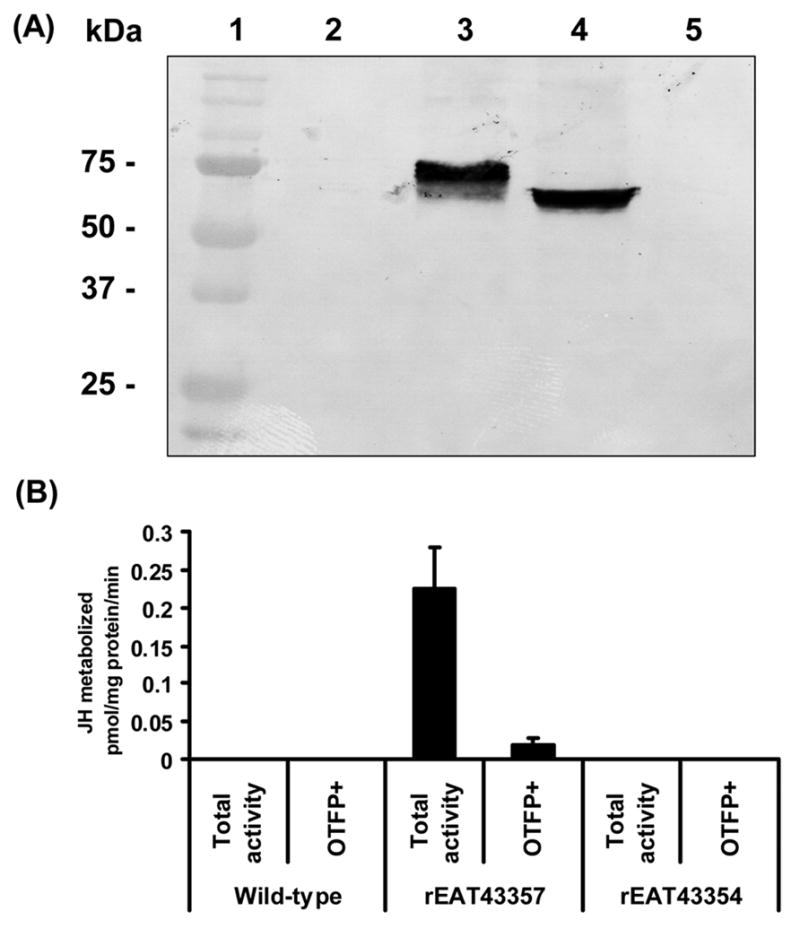
(A) Western blot analysis of the product of EAT43357 cDNA expressed in Sf9 cells. Lane 1: Protein molecular weight marker; Lane 2: Crude protein extract from cells infected with wild-type AcMNPV; Lane 3: Crude protein extract from cells infected with AcMNPV expressing rEAT43357;. Lane 4: Crude protein extract from cells infected with AcMNPV expressing rEAT43354; Lane 5: Crude protein extract from cells infected with AcMNPV expressing rEAT43353. (B) The JHE activity of rEAT43357 protein expressed using the baculovirus system. The total activity bar represents the JHE activity of total proteins, while OTFP+ bar represents the JHE activity when total proteins were incubated with the JHE inhibitor, OTFP. Cell lysates from wild-type baculovirus infected Sf9 cell were used as a negative control. Mean ± SE for three independently expressed recombinant proteins are shown.
4. Discussion
The genome sequence of Ae. aegypti identified 10 genes as those coding for putative jhes. The main purpose of these investigations is to identify Ae. aegypti gene(s) that codes for functional JHE. Sequence analysis showed that not all the putative jhe genes have the five catalytic motifs that have been suggested to be required for H. virescens JHE (HvJHE) activity (Ward et al. 1992). EAT43353, EAT43354 and EAT43357 genes contained most of the conserved catalytic motifs, especially a GQSAG motif. Campbell et.al., (Campbell et al. 2001) reported that the JHE from D. melanogaster and lepidopteran insects contained GQSAG catalytic motif in place of the GESAG motif present in most other esterases. In the D. melanogaster genome two carboxylesterase sequences contain the GQSAG motif, but only one of these two gene products (CG8425) matched the JHE tryptic fingerprint with high confidence (Campbell et al., 2001). The Ae. aegypti genome appears to have at least three carboxylesterases that contain the GQSAG motif. The current study showed that one of them (EAT43357) codes for a functional JHE. Recombinant EAT43354 protein expressed in baculovirus protein expression system showed no JHE activity. We were unable to express the third gene (EAT43357) therefore, whether this gene codes for functional JHE remains unknown. It had been suggested that there may be more than one JHE enzymes in adult D. melanogaster (Campbell et al., 1992; Campbell et al., 1998).
By comparing the developmental expression profiles of JHE transcript and enzyme levels among different insect species, we found that the expression profile of EAT43357 is similar to the JHE mRNA profiles reported from C. fumiferana (Feng et al. 1999) and B. mori (Hirai et al. 2002). In the three insects (C. fumiferana, B. mori and Ae. aegypti) a peak of JHE mRNA was detected during the second half of the final larval instar. Similar to B. mori and M. sexta (Browder et al. 2001), the JHE activity of Ae. aegypti increased gradually during the final instar and reached the maximum level prior to pupation. On the other hand the JHE activity data reported in Culex quinquefasciatus is different from our data. Lassiter et.al., (Lassiter et al. 1995) reported that JHE activity did not change significantly during the fourth larval instar in Cx. quinquefasciatus, and declined in the pupal stage. In D. melanogaster, the mRNA levels of DmJHE started to increase at 36 hr after hatching and showed four peaks at 42 hr (1st instar ), 66 hr (2nd instar), 90 hr (3rd instar) and 132 hr (pupa) after hatching, respectively (Kethidi et al. 2005). The expression profiles of EAT43353 and EAT43354 have some similarity with the expression of DmJHE. Interestingly, only low JHE activity was detected in the final instar larvae of D. melanogaster, while the major peak of JHE occurred 6–12 hr after pupation (Campbell et al. 1992). Comparison of our data from Ae. aegypti with the previous data reported from other insects revealed that EAT43357 may code for a protein with JHE activity. Expression of EAT43357 cDNA in a baculovirus protein expression system followed by JHE enzyme assay using this recombinant protein confirmed that the EAT43357 gene indeed codes for a protein with JHE activity. The mRNA expression of EAT43357 gene correlates well with the JHE activity during the final instar of Ae aegypti, but not with the early pupal stage Considered together, the data presented here show that EAT43357 gene codes for JHE enzyme in Ae. aegypti.
In most insects, the fat body is the primary source of secreted JHE (Wroblewski et al. 1990). The presence of JHE in other tissues such as epidermis has also been reported (Vince and Gilbert, 1977; Mitsui et al., 1979). In Ae. aegypti the expression of jhe gene was detected in the midgut suggesting that the midgut contributes a portion of JHE protein. Moreover, our in situ hybridization detected JHE mRNA in the fat body and midgut. In M. sexta and C. fumiferana, the fat body contained the highest level of the JHE mRNA, while the epidermis and midgut contained lower levels (Wroblewski et al. 1990; Feng et al. 1999). Further investigations are needed to address why JHE is synthesized in multiple tissues, and whether JHE synthesized by the midgut is secreted. In Calpodes ethlius, midgut was shown to secrete proteins both into the lumen and hemolymph (Palli and Locke 1987). Therefore, midgut is capable of synthesizing and secreting proteins into the hemolymph and JHE could be one of those proteins.
JHEs have been identified from D. melanogaster, and several lepidopteran and coleopteran insects. However, JHEs from lower dipterans, especially from important disease vectors have not been identified. Studies reported here on the identification and expression pattern of Aajhe genes will help to understand how the JH titer is regulated during the final instar larval stages of this important disease vector, Ae. aegypti. Recent studies showed that overexpression of jhe caused the precocious metamorphosis of B. mori (Tan et al. 2005). Since most mosquito species are harmful during the adult stages, exploitation of JHE as a target site either by manipulating the expression of this gene through transgenic technology or by developing specific inhibitors may provide novel methods for managing adult mosquito populations. This is especially important in the light of the fact that the most chemical insecticides used for controlling adult mosquitoes work by affecting the nervous system and there are already reports of mosquitoes developing resistance to some of these chemical insecticides by altering the target-site (Weill et al. 2003).
Acknowledgments
This work was supported by the NIH grant RO1 GM070559-02. We thank Dr. Bruce D. Hammock from University of California-Davis for providing JHE inhibitor (OTFP) and Dr. Yiping Li from University of Kentucky for help with promoter analysis. This is contribution number 07-08-038 from the Kentucky Agricultural Experimental Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bonning BC, Hoover K, Booth TF, Duffey S, Hammock BD. Development of a recombinant baculovirus expressing a modified juvenile hormone esterase with potential for insect control. Arch Insect Biochem Physiol. 1995;30:177–194. [Google Scholar]
- Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Browder MH, D’Amico LJ, Nijhout HF. The role of low levels of juvenile hormone esterase in the metamorphosis of Manduca sexta. J Insect Sci. 2001;1:11. [PMC free article] [PubMed] [Google Scholar]
- Campbell PM, Harcourt RL, Crone EJ, Claudianos C, Hammock BD, Russell RJ, Oakeshott JG. Identification of a juvenile hormone esterase gene by matching its peptide mass fingerprint with a sequence from the Drosophila genome project. Insect Biochem Mol Biol. 2001;31:513–520. doi: 10.1016/s0965-1748(01)00035-2. [DOI] [PubMed] [Google Scholar]
- Campbell PM, Healy MJ, Oakeshott JG. Characterization of juvenile hormone esterase in Drosophila melanogaster. Insect Biochem Mol Biol. 1992;22:665–677. doi: 10.1016/s0965-1748(98)00037-x. [DOI] [PubMed] [Google Scholar]
- Campbell PM, Oakeshott JG, Healy MJ. Purification and kinetic characterisation of juvenile hormone esterase from Drosophila melanogaster. Insect Biochemistry and Molecular Biology. 1998;28:501–515. doi: 10.1016/s0965-1748(98)00037-x. [DOI] [PubMed] [Google Scholar]
- Christophers SR. The yellow fever mosquito: its life history, bionomics and structure. Cambridge: The university press; 1960. Aedes aegypti (L) [Google Scholar]
- Feng QL, Ladd TR, Tomkins BL, Sundaram M, Sohi SS, Retnakaran A, Davey KG, Palli SR. Spruce budworm (Choristoneura fumiferana) juvenile hormone esterase: hormonal regulation, developmental expression and cDNA cloning. Mol Cell Endocrinol. 1999;148:95–108. doi: 10.1016/s0303-7207(98)00228-7. [DOI] [PubMed] [Google Scholar]
- Hajos JP, Vermunt AM, Zuidema D, Kulcsar P, Varjas L, de Kort CA, Zavodszky P, Vlak JM. Dissecting insect development: baculovirus-mediated gene silencing in insects, Insect Mol Biol. 1999;8:539–544. doi: 10.1046/j.1365-2583.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- Hammock BD. Regulation of juvenile hormone titer: degradation. In: Kerkut GA, Gilberg LI, editors. Comprehensive insect physiology, biochemistry, and pharmacology, endocrinology. New York: Pergamon Press; 1985. [Google Scholar]
- Hammock BD, Abdelaal YAI, Mullin CA, Hanzlik TN, Roe RM. Substituted thiotrifluoropropanones as potent selective inhibitors of juvenile hormone esterase. Pestic Biochem Physiol. 1984;22:209–223. [Google Scholar]
- Hammock BD, Bonning BC, Possee RD, Hanzlik TN, Maeda S. Expression and effects of the juvenile hormone esterase in a baculovirus vector. Nature. 1990;344:458–461. [Google Scholar]
- Hammock BD, Sparks TC. Rapid assay for insect juvenile hormone esterase activity. Anal Biochem. 1977;82:573–579. doi: 10.1016/0003-2697(77)90196-8. [DOI] [PubMed] [Google Scholar]
- Hanzlik TN, Abdelaal YAI, Harshman LG, Hammock BD. Isolation and sequencing of cDNA clones coding for juvenile hormone esterase from Heliothis virescens. Evidence for a catalytic mechanism for the serine carboxylesterases different from that of the serine proteases. J Biol Chem. 1989;264:12419–12425. [PubMed] [Google Scholar]
- Hanzlik TN, Hammock BD. Characterization of affinity purified juvenile hormone esterase from Trichoplusia ni. J Biol Chem. 1987;262:13584–13591. [PubMed] [Google Scholar]
- Hirai M, Kamimura M, Kikuchi K, Yasukochi Y, Kiuchi M, Shinoda T, Shiotsuki T. cDNA cloning and characterization of Bombyx mori juvenile hormone esterase: an inducible gene by the imidazole insect growth regulator KK-42. Insect Biochem Mol Biol. 2002;32:627–635. doi: 10.1016/s0965-1748(01)00141-2. [DOI] [PubMed] [Google Scholar]
- Kethidi DR, Perera SC, Zheng S, Feng Q-L, Krell PJ, Retnakaran A, Palli SR. Identification and characterization of a JH response region in the juvenile hormone esterasse gene from the spruce, Choristoneura fumiferana. J Biol Chem. 2004;279:19634–42. doi: 10.1074/jbc.M311647200. [DOI] [PubMed] [Google Scholar]
- Kethidi DR, Xi Z, Palli SR. Developmental and hormonal regulation of juvenile hormone esterase gene in Drosophila melanogaster. J Insect Physiol. 2005;51:393–400. doi: 10.1016/j.jinsphys.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Lassiter MT, Apperson CS, Roe RM. Juvenile hormone metabolism during the 4th stadium and pupal stage of the southern house mosquito, Culex quinquefasciatus Say. J Insect Physiol. 1995;41:869–876. [Google Scholar]
- Mitsui T, Riddiford LM, Bellamy G. Metabolism of juvenile hormone by the epidermis of the tobacco hornworm, Manduca sexta. Insect Biochemistry. 1979;9:637–43. [Google Scholar]
- Nishiura JT. Coordinated morphological changes in midgut, imaginal discs and respiratory trumpets during metamorphosis of Aedes aegypti (Diptera: Culicidae) Ann Entomol Soc Am. 2002;95:498–504. [Google Scholar]
- Palli SR, Locke M. Hemolymph protein synthesis by the larval midgut of an insect Calpodes ethlius (Lepidoptera: Hesperiidae) Insect Biochem. 1987;17:561–572. [Google Scholar]
- Riddiford LM. Juvenile hormone: the status of its “status quo” action. Arch Insect Biochem Physiol. 1996;32:271–286. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<271::AID-ARCH2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Share MR, Roe RM. A partition assay for the simultaneous determination of insect juvenile hormone esterase and epoxide hydrolase activity. Anal Biochem. 1988;169:81–88. doi: 10.1016/0003-2697(88)90257-6. [DOI] [PubMed] [Google Scholar]
- Shiotsuki T, Bonning BC, Hirai M, Kikuchi K, Hammock BD. Characterization and affinity purification of juvenile hormone esterase from Bombyx mori. Biosci Biotechnol Biochem. 2000;64:1681–1687. doi: 10.1271/bbb.64.1681. [DOI] [PubMed] [Google Scholar]
- Tan A, Tanaka H, Tamura T, Shiotsuki T. Precocious metamorphosis in transgenic silkworms overexpressing juvenile hormone esterase. Proc Natl Acad Sci U S A. 2005;102:11751–11756. doi: 10.1073/pnas.0500954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BA, Hinton AC, Moskowitz H, Severson TF, Hammock BD. Isolation of juvenile hormone esterase and its partial cDNA clone from the, Tenebrio molitor. Insect Biochem Mol Biol. 2000;30:529–540. doi: 10.1016/s0965-1748(00)00020-5. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Abdelaal YAI, Armstrong FB, Roe RM. Characterization of affinity purified juvenile hormone esterase from the plasma of the tobacco hornworm, Manduca sexta. J Biol Chem. 1990;265:21727–21732. [PubMed] [Google Scholar]
- Vermunt AMW, Koopmanschap AB, Vlak JM, De Kort CAD. Cloning and sequence analysis of cDNA encoding a putative juvenile hormone esterase from the Colorado potato beetle. Insect Biochem Mol Biol. 1997a;27:919–928. doi: 10.1016/s0965-1748(97)00073-8. [DOI] [PubMed] [Google Scholar]
- Vermunt AMW, Vermeesch AMG, deKort CAD. Purification and characterization of juvenile hormone esterase from hemolymph of the Colorado potato beetle. Arch Insect Biochem Physiol. 1997b;35:261–277. doi: 10.1002/(SICI)1520-6327(199705)35:3<261::AID-ARCH2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Vince RK, Gilbert LI. Juvenile hormone esterase activity in precisely timed last instar larvae and pharate pupae of Manduca sexta. Insect Biochemistry. 1977;7:115–20. [Google Scholar]
- Ward VK, Bonning BC, Huang T, Shiotsuki T, Griffeth VN, Hammock BD. Analysis of the catalytic mechanism of juvenile hormone esterase by site-directed mutagenesis, Int J Biochem. 1992;24:1933–1941. doi: 10.1016/0020-711x(92)90289-d. [DOI] [PubMed] [Google Scholar]
- Weill M, Lutfalla G, Mogensen K, Chandre F, Berthomieu A, Berticat C, Pasteur N, Philips A, Fort P, Raymond M. Comparative genomics: Insecticide resistance in mosquito vectors, Nature. 2003;423:136–137. doi: 10.1038/423136b. [DOI] [PubMed] [Google Scholar]
- Wogulis M, Wheelock CE, Kamita SG, Hinton AC, Whetstone PA, Hammock BD, Wilson DK. Structural studies of a potent insect maturation inhibitor bound to the juvenile hormone esterase of Manduca sexta. Biochemistry. 2006;45:4045–4057. doi: 10.1021/bi0521644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewski VJ, Harshman LG, Hanzlik TN, Hammock BD. Regulation of juvenile hormone esterase gene expression in the tobacco budworm (Heliothis virescens) Arch Biochem Biophys. 1990;278:461–466. doi: 10.1016/0003-9861(90)90285-7. [DOI] [PubMed] [Google Scholar]
- Wu Y, Parthasarathy R, Bai H, Palli SR. Mechanisms of midgut remodeling: Juvenile hormone analog methoprene blocks midgut metamorphosis by modulating ecdysone action, Mech Dev. 2006;123:530–547. doi: 10.1016/j.mod.2006.05.005. [DOI] [PubMed] [Google Scholar]


