Abstract
We have combined anatomical and functional methodologies to provide a comprehensive analysis of the properties of nicotinic acetylcholine receptors (nAChRs) on developing dopamine (DA) neurons. Double-labeling in situ hybridization was used to examine the expression of nAChR subunit mRNAs within developing midbrain DA neurons. As brain maturation progressed there was a change in the pattern of subunit mRNA expression within DA neurons, such that α3 and α4 subunits declined and α6 mRNA increased. Although there were strong similarities in subunit mRNA expression in substantia nigra (SNc) and ventral tegmental area (VTA), there was higher expression of α4 mRNA in SNc than VTA at gestational day (G)15, and of α5, α6 and β3 mRNAs during postnatal development. Using a superfusion neurotransmitter release paradigm to functionally characterize nicotine-stimulated release of [3H]DA from striatal slices, the properties of the nAChRs on DA terminals were also found to change with age. Functional nAChRs were detected on striatal terminals at G18. There was a decrease in maximal release in the first postnatal week, followed by an increase in nicotine efficacy and potency during the second and third postnatal weeks. In the transition from adolescence (postnatal days (P) 30 and 40) to adulthood, there was a complex pattern of functional maturation of nAChRs in ventral, but not dorsal, striatum. In males, but not females, there were significant changes in both nicotine potency and efficacy during this developmental period. These findings suggest that nAChRs may play critical functional roles throughout DA neuronal maturation.
Keywords: adolescent, fetal, nicotine, sex, striatum, maternal smoking
Maternal smoking during pregnancy has been correlated with a number of adverse outcomes in the offspring (Lichtensteiger et al., 1988, Lichtensteiger and Schlumpf, 1993), including cognitive deficits that are often manifested in early childhood as attention deficit hyperactivity disorder (ADHD) (Millberger et al., 1996). Though the underlying neurobiological mechanism responsible for this disorder has been a matter of some controversy, alterations in development of central DA systems have been implicated (Lichtensteiger et al., 1988, Pliszka et al., 1996, Castellanos, 1997). In animal studies, maternal nicotine exposure produces sex-dependent alterations in the development of DA neurochemical markers (Fung, 1988, Lichtensteiger et al., 1988, Fung, 1989, Ribary and Lichtensteiger, 1989, Lichtensteiger and Schlumpf, 1993, Muneoka et al., 1997), and induces hyperactivity in the offspring, which is believed to result from alterations in mesolimbic and nigrostriatal DA systems (Schlumpf et al., 1988, Fung and Lau, 1989, Richardson and Tizabi, 1994, Tizabi et al., 1997, Ajarem and Ahmad, 1998). However, one point of intense controversy is whether the actions of nicotine are mediated directly on DA neurons or are the result of indirect effects such as hypoxia (Slotkin, 1998).
Adult midbrain DA neurons express a variety of nAChR subunit mRNAs, including α3-α7, β2 and β3 (Charpantier et al., 1998, Elliott et al., 1998, Sorenson et al., 1998, Klink et al., 2001, Azam et al., 2002). Nicotine has been shown to directly activate these neurons (Pidoplichko et al., 1997, Yin and French, 2000). Moreover, nicotine stimulates release of DA within the striatal target region by direct action on nicotinic receptors on DA terminals (Rapier et al., 1990, Grady et al., 1992, Clarke and Reuben, 1996, Wonnacott, 1997). Although this system has been extensively studied in the adult, not much is currently known about the properties of nAChRs within developing midbrain DA neurons.
The present study combined anatomical and functional methodologies to provide an extensive ontogenetic analysis of mRNA and protein expression, as well as functional development of nAChRs, within midbrain DA neurons. This study was undertaken to 1) determine if there are functional nAChRs in the fetus that can directly mediate effects of prenatal nicotine exposure on the development of midbrain DA neurons, 2) examine properties of nAChRs during the brain growth spurt, another period of nicotine susceptibility, which spans approximately the first 3 postnatal weeks in rats and corresponds to third trimester-2nd year of human development, 3) compare the functional properties of nAChRs on dorsal and ventral striatal DA terminals between adolescent and adult rats and 4) examine possible sex differences in nAChR properties from birth until adulthood. We have used a double-labeling technique to examine nAChR subunit transcript expression within DA neurons. Nicotine-stimulated [3H]DA release from striatal slices was also used to examine the functional status of nAChRs on DA terminals throughout development.
MATERIALS AND METHODS
Materials
The following materials were obtained from the indicated sources: bovine serum albumin, polyvinylpyrrolidone, poly-L-lysine, RNase A, (−)nicotine bitartarate, D(+)-glucose and HEPES (Sigma-Aldrich, St. Louis, MO, USA); pBluescript II SK+ (Stratagene, La Jolla, CA, USA); anti-Digoxygenin (Dig)-AP Fab antibody, Dig-UTP, Genius system nonradioactive nucleic acid detection kit, restriction enzymes, T3, T7 polymerases, proteinase K and yeast tRNA (Boehringer Mannheim Biochemicals, Indianapolis, IN); formamide (Fluka, Ronkonkoma, NY, USA); dextran sulfate (Pharmacia, Piscataway, NJ, USA); Hyperfilm, Bmax (Amersham, Arlington Heights, IL, USA); nuclear track emulsion (NTB2) (Kodak, Rochester, NY, USA); 35S-Uridine triphosphate (35S-UTP) and [3H]dihydroxyphenylethylamine (DA) (specfic activity: 20-40 Ci/mmol) (New England Nuclear, Boston, MA, USA). Ecolume scintillation cocktail (MP Biomedical, Solon, OH). All other chemicals were purchased from Fisher Scientific (Pittsburg, PA, USA).
Animals
Pregnant Sprague Dawley rats (Harlan, San Diego, CA) were maintained in a temperature (21°C) and humidity (50%) controlled room on a 12-h light-dark cycle (lights on 0700-1900) with unlimited access to food and water. All experiments were carried out in accordance with the Institutional Animal Care and Use Committee at the University of California, Irvine, and were consistent with Federal guidelines. Some dams were killed by decapitation at gestational day (G)15 and G18 and their fetuses harvested by caesarian section. Other dams were allowed to deliver, with the day of birth designated as P0. Litters were culled to ten pups after birth to ensure adequate maternal care, and were weaned at P21 and group housed with littermates of the same sex. Only one pup of each sex per litter was assigned to an experimental group for anatomical studies. For neurotransmitter release, striata of several pups of a given sex from a single litter were pooled to ensure adequate tissue for the study.
Double-labeling in situ hybridization
Tissue preparation
Males and females, aged G15, G18, P1, P4, P7, P14, P21, P30, P40 and adult (P60 and older) were decapitated and brains immediately removed and frozen in −20°C isopentane. The frozen brains were stored at −70°C until use. Twenty-micron sections were cryostat cut and mounted onto slides, which were coated with gelatin and poly-L-lysine and kept at −20°C. Sections were postfixed with 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4, for 1 hr at 22°C, then washed in PB, air dried, and stored desiccated at −20°C until use.
cRNA probe preparation
The cDNAs (length in base pairs) were kindly provided by Dr. J. Boulter (UCLA, Los Angeles, CA). α3 (1858) and β3 (1780) were subcloned in pBluescript (pBS) II SK+, between EcoRI and HindIII and KpnI and EcoRI sites, respectively. The remaining clones are as indicated: α4-1 (2110), cloned in pSP64 in the HindIII site, α5 (1607) , α6 (1760), cloned in pBS SK(−) in the EcoRI site, α7 (2100), β2 (2196), cloned in pSP65 in the EcoRI site, and β4 (2522), cloned in pBS SK(−) in the EcoRI site. Plasmids were linearized with the appropriate restriction enzyme and 35S-labeled riboprobes were synthesized in antisense and sense orientations by using 35S-UTP, according to the method of Simmons et al., 1989. All probes were further subjected to alkaline hydrolysis to yield products with an average size of 600 bases according to Cox et al., 1984. Nonradioactive Dig-labeled cRNA probe for tyrosine hydroxylase (TH; 230 base pairs) was synthesized by using Dig-labeled UTP and appropriate transcription enzyme. The concentrations of the Dig-labeled probes were determined by dot blotting. The specificity of the cRNA probes used in the present study was extensively examined in Azam et al., 2002.
TH Dig/nAChR subunit mRNA colocalization
Tissue sections were processed for double labeling in situ hybridization as described previously (Winzer-Serhan and Leslie, 1997). Briefly, sections were pretreated with proteinase K (1 μg/ml) for 10 min at 22°C, acetylated, dehydrated through graded ethanol (50, 70, 95 and 100%) and air-dried. Sections were then incubated for 18 hours at 60°C with 1:1 dilution of Dig-labeled antisense TH riboprobe (0.1 μg/ml): 35S-labeled sense or antisense nAChR subunit probes (2 × 107 cpm/ml) in hybridization solution (50% formamide, 10% dextran sulfate, 0.02% Ficoll, 0.02% polyvinyl pyrolidone, 0.02% bovine serum albumin, 500 μg/ml tRNA, 10 mM dithiothreitol, 0.3 M NaCl, 10 mM Tris, pH 8.0, 1 mM EDTA, pH 8.0). After hybridization, sections were incubated with RNase A (20 μg/ml) for 30 min at 37°C, followed by two 5-min and two 10-min high stringency washes of decreasing salinity (2x-0.5x standard saline citrate (SSC) buffer) at 22°C and a 30 min wash in 0.1x SSC at 65°C. After the hot wash, the slides were incubated in Genius buffer 1 (GB1) (100 mM Tris-HCl, 150 mM NaCl, pH 7.5) for 1 minute, followed by a 30-min wash in 5% non-fat dry milk in GB1 + 0.25% Triton-X (GB2) at 22°C. The anti-Dig alkaline phosphatase conjugated Fab antibody (sheep), prepared as 1:5000 dilution in GB2, was applied by drop technique and slides incubated for 3 hrs at 37°C. The slides were washed three times for 1, 5 and 10 min in GB1 + 0.25% Triton-X. Color reagent, composed of 50 μl NBT and 37.5 μl BCIP in 10 ml GB3 (100 mM Tris-HCl, 100 mM NaCl, 50 mM MgCl2, pH 9.5) was applied and slides incubated overnight at 22°C. The next day, the slides were washed twice in GB4 (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and once in double deionized water, dehydrated with brief dips in graded ethanol (50, 70, 95 and 100%), air dried and apposed to β-max film for an appropriate period of time. Following film development, slides were coated with 3% parlodion in isoamylacetate and dipped in liquid NTB2 emulsion on the reference date of the 35S-UTP. After the appropriate exposure period, slides were developed in Kodak developer D-19, fixed, coverslipped and analyzed.
Data Analysis
For each subunit, data from male and female rats were pooled to yield an n = 2 for developmental ages G15-P10. For α5, α6, α7 and β3 subunits, an additional 2 animals per sex were prepared for ages P14, P21, P30, P40 and adult. Since there were no major sex differences in subunit expression at any of these ages, all data for males and females were pooled. For each animal, at least 2 sections per subunit were analyzed and averaged. The data presented for each subunit and each age are means ± SEM from all animals (males and females).
The hybridization signal for each nAChR subunit riboprobe was quantified on the autoradiographic films using a video-based computerized image analysis system (MCID, Image Research Inc., St. Catharines, Ontario, Canada). The total optical density of the hybridization signal was measured in the SNc and the VTA, as identified by digoxigenin-labeled TH cells on the corresponding emulsion-dipped slide, as well as identifiable landmarks, such as interpduncular nucleus and/or medial terminal nucleus of accessory optical tract (which visibly separated SNc from the VTA in some sections) (Paxinos and Watson, 1986), to delimit the VTA. The radioactivity values were determined by extrapolation from a standard curve generated from 14C brain paste standards and expressed as dpm/mg of tissue. Non-specific signal, as determined from the sense probe, was ≤ 5%, as has been shown in previous studies from our laboratory (Winzer-Serhan and Leslie, 1997, Azam et al., 2002).
Statistical analysis was performed with Graphpad Prism (San Diego, CA). Differences in expression of α5, α6 and β3 subunits between the SNc and the VTA across development were first analyzed by two-way ANOVA. If the analysis yielded significance across age and/or area, further post-hoc tests were performed. For analysis between areas, multiple Student's t-test with Bonferroni correction for multiple comparisons was used. For analysis across development (P14 to P60), data in either the SNc or the VTA were further analyzed by one-way ANOVA, followed by Dunnett's post-hoc with expression at P60 as control.
Coexpression of nAChR subunit mRNAs and TH-labeled DA neurons was determined by light microscopic analysis using an Olympus BX50 microscope (Scientific Instruments, Temecula, CA) and transillumination system (Micro Video Instruments, Inc., Avon, MA). 35S-labeled cells expressing a given nAChR subunit mRNA were identified by silver grain counts at least threefold over background, as determined by averaging the number of grains over 20 digoxigenin-labeled cells in the corresponding sense slide (Winzer-Serhan and Leslie, 1997, Azam et al., 2002). Double-labeled cells were identified as cells expressing TH mRNA (cell-sized deposits of visible digoxigenin reaction product) and nAChR subunit mRNA (silver grain counts more than threefold over background). Photographic images were created by a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI) and corrected for brightness and contrast using Adobe Photoshop 5.5.
Ligand binding autoradiography
Tissue Preparation
For binding experiments, brains were cut at the same developmental ages as were used the in situ hybridization experiments. Twenty-micron coronal sections were mounted onto gelatin-coated slides, dried with desiccant at 4°C for 2 hr and then stored at −20°C until use.
Ligand binding
For [3H]nicotine (NIC) and [125I]α-bungarotoxin (BTX) binding, slides were pre-incubated in the appropriate pre-incubation buffer for 15 min at room temperature (RT) (for [3H]NIC labeling: 50 mM Tris hydrochloride, 8 mM CaCl2, pH 7.4, supplemented with 0.005% PEI; for [125I]α-BTX labeling: 50 mM Tris hydrochloride, 120 mM NaCl, pH 7.4). For [3H]nicotine binding, 1 ml of buffer containing 10 nM radioligand was applied to each slide and incubated for 20 min at RT. For non-specific binding, 10 μM unlabeled nicotine was also added to the incubation buffer. After incubation, slides were washed by 3 × 10 sec dips in buffer and 1 dip in dH2O, all at 4°C. For [125I]α-BTX binding, 1 ml of buffer containing 5 nM radioligand was applied to each slide and incubated for 2 hr at RT. For non-specific binding, 10 μM α-cobratoxin was added to the incubation mixture. The slides were then rinsed 2 × 10 min in buffer and 2-3 dips in dH2O, all at 4°C. After the washing step, slides were air dried for 1 hr and exposed with appropriate standards of known radioactivity to β-max film ([125I]α-BTX) or Hyperfilm ([3H]NIC). After an appropriate exposure period, films were developed, and sections post-fixed with formaldehyde and stained with Cresyl violet for identification of anatomical structures.
Data analysis
Autoradiograms were quantified as described above for the in situ hybridization, except that the calibration curve of optical density against radioligand concentration was constructed to reflect fmol/mg tissue. The curve was constructed using [14C] brain paste standards of known radioactivity and calibrated for reading [125I] emissions, as described by (Miller and Zahniser, 1987). For tritium binding, standard curves were constructed using [3H] standards. Optical densities in discrete regions of autoradiographic images were measured, using nissl stained and TH-Dig labeled sections as an anatomical guide, and corresponding values of radioactivity were determined by interpolation from the standard curve. For each age, the corresponding non-specific signal, as determined from the binding in presence of excess unlabeled ligand, was subtracted from the total binding to obtain specific levels. At least 2 sections per animal per radioligand were analyzed and averaged. Average specific binding across all ages was 77 ± 2% for [3H]NIC and 62 ± 4% for [125I]α-BTX.
Neurotransmitter release assay
Tissue Preparation
Male and female Sprague Dawely rats, aged G17-18, P1, P4, P7, P14, P21 P30, P40 and adult (P60 and older), were killed by decapitation and brains quickly removed. For G18 fetal rats, a coronal section approximately 2 mm thick (after removal of prefrontal cortex) was cut and the tissue adjacent to the ventricles, minus the developing cortex, was removed from both sides. For early postnatal timepoints, a coronal section was cut at the level of striatum and whole striatum was removed bilaterally. For adolescent and adult animals, a transverse cut was made to separate dorsal and ventral striata. For fetal ages, tissue from males and females were combined for each experiment, whereas for older ages, striata from 2-6 animals per sex were pooled. The striata were cross-chopped into 250 μm slices by a McIlwain tissue chopper. The slices were washed 2 × 5min and 1 × 10 min in Krebs-HEPES buffer (127 mM NaCl, 5 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, 15 mM HEPES acid, 10 mM glucose, 1 mM ascorbic acid, pH 7.4) at 37°C, then incubated for 20 min in buffer containing 50 nM [3H]DA. Slices were then washed 2 × 5 min and 2 × 10 min to remove excess radioactivity.
Superfusion release assay
After the washes, 15 μl aliquots of gravity packed slices were transferred to each chamber of a Brandel superfusion apparatus. Slices were superfused at a rate of 0.4 ml/min with Krebs-HEPES buffer at room temperature. After a 36-min wash and stabilization period, four 2.5-min fractions were collected to determine basal tritium outflow. After the basal collection, a 1-min pulse of various doses of nicotine (100 nM-30 μM) was applied, followed by collection of nine 2.5-min fractions. A 1-min pulse of 25 mM K+ Krebs-HEPES buffer (with NaCl concentration decreased accordingly to maintain osmolarity) was then applied to ensure tissue viability, followed by collection of three 2.5-min fractions. At the conclusion of the superfusion, slices in each chamber were transferred to a vial containing 2 ml 5% HCl/95% EtOH and lysed for 30 min. After addition of 5 ml of Ecolume scintillation fluid to each vial, they were counted in a Beckman Liquid Scintillation Counter, model 5801.
Data analysis
Percent fractional efflux of [3H]DA was calculated as the amount of radioactivity in the superfusate fraction relative to the total amount taken up by the slices. Basal release was calculated by taking the mean of one pre-stimulus fraction immediately preceding agonist application and one post-stimulus fraction upon return to baseline levels. Evoked [3H]DA release was calculated as area under the curve after subtraction of the average baseline. Data from all animals per sex at each developmental time-point (except fetal, where males and females were not separated) were pooled together and analyzed. Dose-response curves were constructed by non-linear regression with maximum release (average of maximum release at each age) set as a constant in order to obtain the best fit curve and allow calculation of EC50s (GraphPad Prism, San Diego, CA). Differences were first analyzed by two-way ANOVA; if significance was obtained, further analysis was carried out by one-way ANOVA, followed by Dunnett's post-hoc with P21 (for G17-18 to P14) or adult (for P30 & P40) as controls. Sex differences in maximum release values were determined by Student's t-test, with Bonferroni correction for multiple comparisons.
RESULTS
nAChR subunit mRNA expression within SNc and VTA
Fetal period
Most of the subunits that are known to be expressed within the adult SNc and VTA DA neurons (Azam et al., 2002) were also present at G15, the earliest timepoint examined. At this early age α3, α4, α5 and β2 mRNAs were expressed at higher levels than in the adult (Fig. 1). As can be seen in the emulsion-dipped slides, which allow analysis at the neuronal level (Fig. 2), these nAChR subunits were co-expressed in TH neurons as early as G15. Furthermore, there was nAChR subunit expression in what appeared to be cells that were migrating to the SNc and VTA, but which were not yet TH positive. There was a striking regional difference in expression of α4 mRNA at G15, with markedly higher levels in the SNc than the VTA. This difference in regional expression was restricted to α4 mRNA and was not seen for other subunits (Fig. 1). By G18, α4 mRNA within the SNc had declined to levels similar to that of the VTA (Fig. 1). The decline that occurred in α4 mRNA levels within SNc at G18 was most likely due to a true down-regulation of mRNA levels, since the cell packing density is not much different between G15 and G18. However, since there was a widespread distribution of α4 mRNA in both the DA and non-DA cells (Fig. 2), it was difficult to determine whether this downregulation occurred in DA, non-DA or both neuronal types. The levels of α4 mRNA in the SNc and the VTA did not change markedly from G18 to adulthood (Fig. 1).
Figure 1.

Developmental profiles of α3, α4, α5, α6, α7, β2, β3 and β4 mRNAs within SNc and VTA. Negative values on the x-axis indicate prenatal periods G15 (−7) and G18 (−4). P0 indicates birth (G22). Values represent mean ± SEM from 2-10 animals (males and females). *p<0.05, **p<0.01, ***p<0.001, significantly different from the VTA, t-test with Bonferroni correction. ###p<0.001, significantly different from adult, Dunnett's post-hoc.
Figure 2.

Photomicrographs of emulsion-dipped slides showing expression of α3, α4, α5, α6, α7, β2, β3 and β4 mRNAs (silver grains) in SNc and VTA at G15, P1, P21 and P60. Dark cells represent TH Dig -labeled DAergic neurons. In all panels, SN is on the left hand side and VTA on the right. Scale bar = 200μm.
Expression levels of α3, α5 and β2 mRNAs, which were high at G15 in both SNc and VTA, also declined until birth. In contrast, the moderately low expression of α7 mRNA remained unchanged. There was low expression of α6 mRNA within DA cells of both SNc and the VTA at G15, with a slight increase at G18 (Fig. 1). Prior to birth, β3 mRNA levels in DA cells were similar to that of the adult, but with no difference in expression between SNc and VTA. Although there was significant expression of β4 mRNA within SNc and VTA during the perinatal period, microscopic analysis of the emulsion-dipped slides indicated that this was restricted to non-DA neurons within both SNc and VTA (Fig. 2). (Figures 1 & 2).
Postnatal period
After birth, there was an increase in the expression of α6 mRNA, which was expressed at low levels in fetal SNc and VTA (Fig. 1). There was also a secondary increase in the expression of α5 and α7 subunit mRNAs. Two-way ANOVA revealed both regional and age differences in the expression of α5 and α6 mRNAs during the postnatal period (α5: Age, F(4,78)=8.968, p<0.001; Area, F(1,78)=25.95, p<0.001; α6: Age, F(4,86)=10.53, p<0.001; Area, F(1, 86)=46.96), p<0.001) (Fig. 1). Post-hoc analyses revealed that α5 subunit transcript levels in the SNc were significantly higher at P21 as compared to the adult, whereas in the VTA levels were significantly higher at P14 and P21 as compared to the adult (Fig. 1). Furthermore, α5 mRNA levels were significantly higher in SNc than in VTA at both P30 and P40 (Fig. 1). For the α6 subunit, mRNA levels in both the SNc and the VTA were significantly higher at P21than in adult. Regional differences in α6 transcript levels were also observed, with significantly higher expression in the SNc than the VTA at P21 and P40 (Fig. 1). As with α5 and α6 subunits, expression of the α7 mRNA also peaked at the end of the third postnatal week and declined to lower adult levels (Fig. 1); however, no regional differences in expression were observed. Double-labeling in situ hybridization experiments confirmed that the peak in mRNA expression levels at P21 largely reflected increased transcript levels in individual DA neurons, as evidenced by higher numbers of silver grains per DA cell, rather than increased expression in non-DA cells (Fig. 3).
Figure 3.
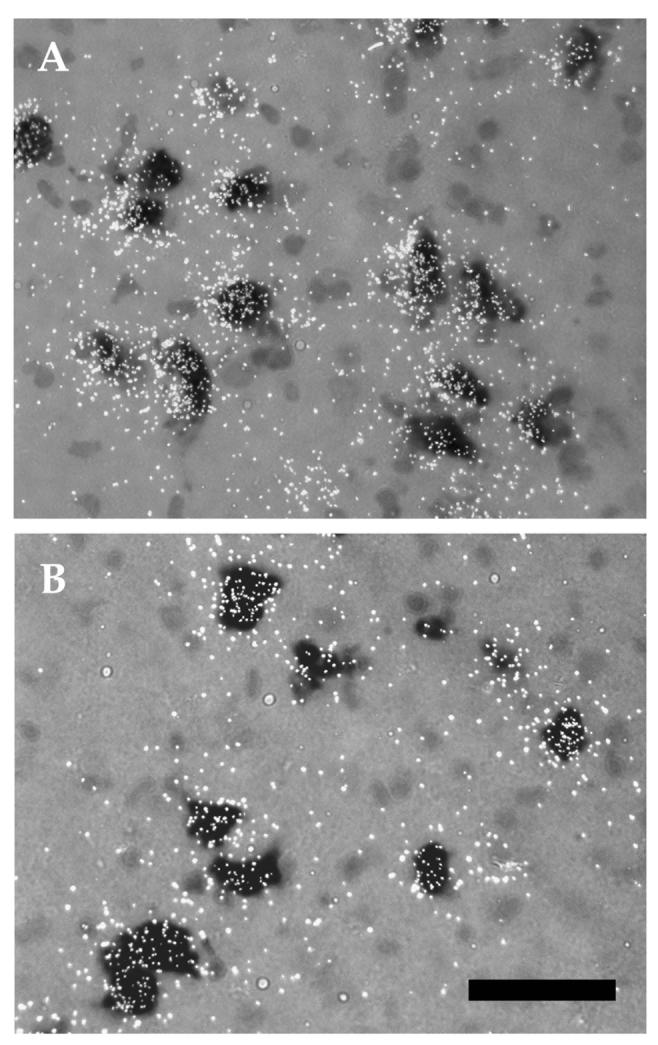
Photomicrographs of α6 mRNA expression within midbrain DA neurons at P21 (A) and adult (B). Note the higher number of silver grains per DA neuron (dark cells) in P21 as compared to the adult. The peak of expression of this subunit at P21 is, therefore, due to higher level of mRNA per DA neuron. Scale bar = 50μm.
Although β3 mRNA levels in the VTA showed a slight decline at birth, levels in both SNc and VTA remained relatively constant throughout development after birth. However, significant regional differences in β3 mRNA expression were evident during the postnatal period (F(1,70) = 21.42, p<0.001). At P60, this subunit displayed significantly higher expression in the SNc than the VTA (Fig. 1).
Developmental profile of nAChR radioligand binding within SNc and VTA
As with the mRNA data, the results from males and females were pooled for the binding analysis since no major sex differences were apparent (Fig. 4). Alternate brain sections were processed for TH-Dig and binding with two different nAChR ligands, [3H]NIC and [125I]α-BTX. [3H]NIC binds to the high affinity α4β2 receptors (Perry and Kellar, 1995). [125I]α-BTX recognizes one class of nAChRs containing the α7 subunit (Chen and Patrick, 1997, Drisdel and Green, 2000).
Figure 4.
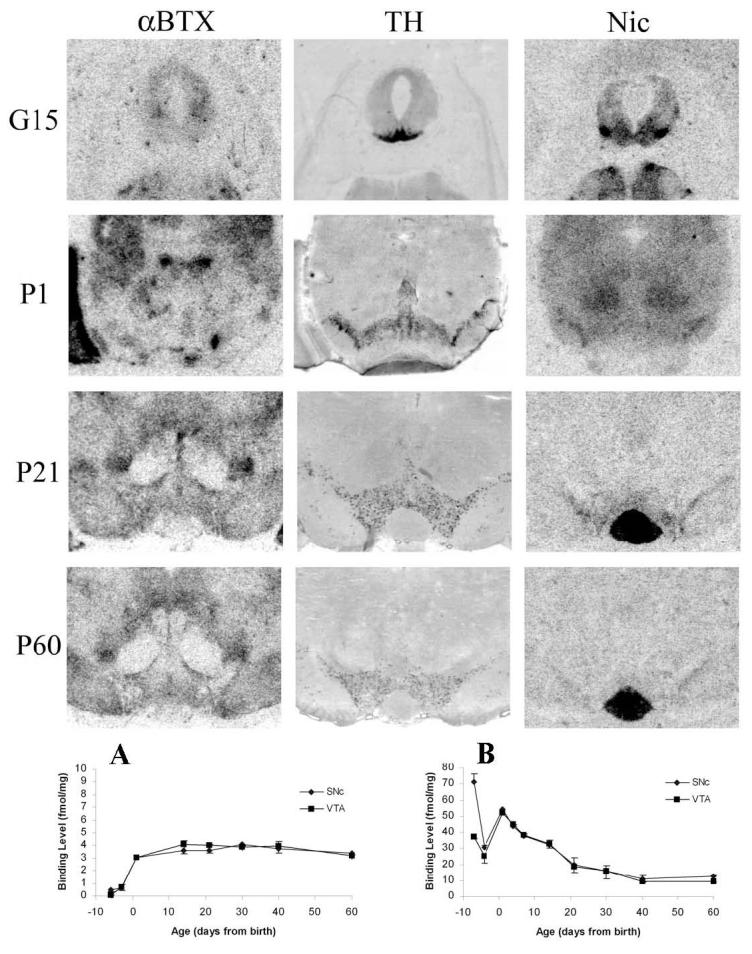
Autoradiograms and developmental profiles of (A) [125I]α-BTX and (B) [3H]NIC binding within midbrain DA areas. Dig-labeled TH labeling (middle panel) is shown to indicate areas analyzed for binding. Values for [3H]NIC and [125I]α-BTX represent mean ± SEM from one male and one female per age.
The binding of each ligand exhibited a distinct developmental profile. [3H]NIC binding was high during the prenatal period, with higher levels in the SNc than VTA at G15 (Fig. 4A), a regional difference which disappeared by G18. The differential expression of [3H]NIC in SNc and VTA at G15, and its decline in SNc by G18, paralleled that of α4 mRNA expression (see Fig. 1). However, developmental profiles of [3H]NIC binding and α4 mRNA expression diverged at birth; whereas α4 mRNA levels remained unchanged from G18 until adulthood, [3H]NIC binding increased at birth and then slowly declined to adult levels by P40. (Figure 4).
There was no specific binding of [125I]α-BTX in either SNc or VTA at G15 (Fig. 4B). There was an increase in binding levels immediately after birth, which remained constant until adulthood. The developmental profile of the [125I]α-BTX binding weakly correlated with that of α7 mRNA (Fig. 1).
Nicotine-stimulated [3H]DA release from striatal slices
Fetal and early postnatal period
Comparison of P21 and adult animals did not reveal significant differences in either maximal release or EC50 values (data not shown). Therefore, release data from prenatal and early postnatal periods were compared to P21. During the prenatal and early postnatal periods, there was significantly less [3H]DA uptake by striatal slices at G17-18, and more uptake at P4, as compared to P21 (Fig. 5A). However, the developmental variability in uptake was controlled for since release at each age was normalized to total uptake. K+-stimulated release was significantly lower at G17-18 and during the first postnatal week as compared to P21, indicating decreased excitability of DA terminals to depolarizing stimuli during early development (Fig. 5B). (Figure 5)
Figure 5.
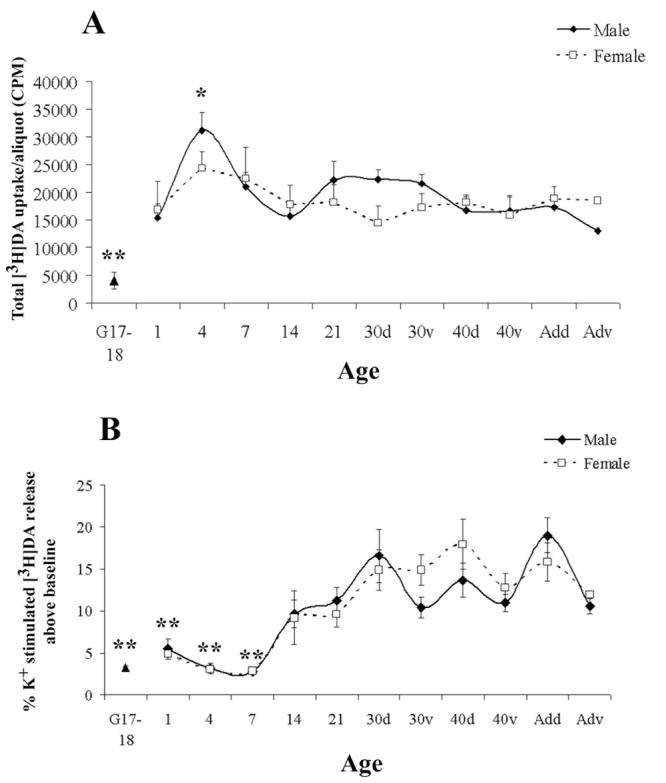
Developmental profile of (A) average total [3H]DA uptake and (B) 15 mM K+-induced [3H]DA release throughout development. Data from males and females are shown separately for ages P1-60. Because males and females were pooled at G17-18, values are shown as a single point. Values are mean ± SEM from 3-7 independent experiments. *p<0.05, **p<0.01, significantly different from P21 using Dunnett's post-hoc analysis.
Nicotine dose-dependently stimulated [3H]DA release from striatal slices at all ages. However, during the fetal and neonatal periods, only the higher concentrations of nicotine stimulated detectable release (Fig. 6A). Statistical comparison of nicotine EC50 values showed significant age and sex differences (p<0.0001, F(4,45)=14.71 and p<0.05, F(1,45)=7.184, respectively). Nicotine was significantly less potent at G17-18, in females at P1 and in both sexes at P7, as compared to later postnatal ages (Table 2). Maximal nicotine-stimulated [3H]DA release also changed significantly with age (p<0.0001, F(4,24)=14.15). Whereas nicotine efficacy at G17-18 was similar to that at P21, maximal nicotine-stimulated release declined at P1 and remained lower for the next two postnatal weeks (Table 1). (Table 1 & Figure 6)
Figure 6.
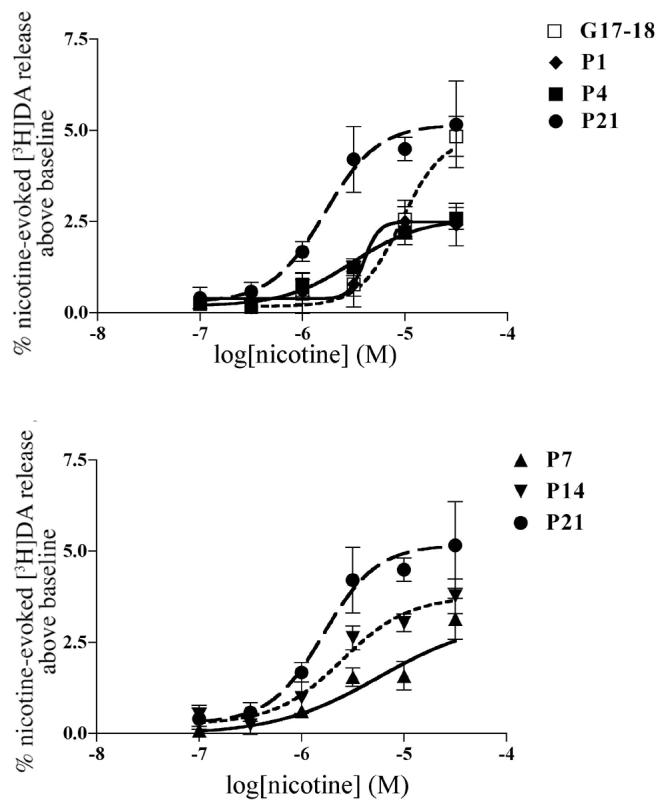
Nicotine-stimulated [3H]DA release from males during the first 3 postnatal weeks. (A) nicotine-stimulated [3H]DA release from prenatal and perinatal striatum. (B) Nicotine stimulated [3H]DA release at end of first and second postnatal weeks. Release from P21 animals is shown for comparison. At all postnatal ages, maximum release is significantly lower than at P21, a developmental period when nicotine-stimulated [3H]DA release has reached adult levels Values are mean ± SEM from 3-4 independent experiments.
Table 2.
Nicotine-stimulated [3H]DA release from dorsal striatum of male and female animals. EC50s (95% confidence intervals) and maximum release (mean ± SEM) are from 3-6 independent experiments.
| Age | EC50 (μM) | Max. Release (%) | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 30 | 3.26 (2.3-4.6) |
2.14 (0.7-6.7) |
7.7 ± 0.26 | 4.87 ± 1.01 |
| 40 | 1.42 (0.3-6.4) |
3.9 (1.5-9.9) |
6.60 ± 1.00 | 7.39 ± 1.32 |
| Ad | 2.55 (0.9-7.1) |
2.05 (0.7-6.1) |
6.39 ± 0.62 | 7.33 ± 0.85 |
Table 1.
Nicotine-induced [3H]DA release during the prenatal period and first 3 postnatal weeks. EC50 values (95% confidence intervals) and maximum release (mean ± SEM) are from 3-4 independent experiments. [3H]DA release was measure from the whole striatum.
| Age | EC50 (μM) | Max. Release(%) | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| G17-18 | 9.2 *** (5.7-15) | 4.84±0.55 | ||
| 1 | 4.0 (0.4-42) |
11.33*** (8-16) |
2.50±0.58*** | 3.32±0.76* |
| 4 | 3.09 (1.5-6.6) |
3.12 (0.8-12) |
2.59±0.30*** | 1.63±0.44*** |
| 7 | 5.78*** (2.0-17) |
8.67*** (4.5-17) |
3.15±0.56*** | 2.64±0.40*** |
| 14 | 2.48 (1.3-4.8) |
2.52 (1.1-5.5) |
3.76±0.48* | 4.75±0.30 |
| 21 | 1.65 (0.8-3.3) |
2.35 (0.72-7.7) |
6.19±0.86 | 6.34±1.10 |
p<0.05
p<0.001
values significantly different from P21 of the same sex, Dunnett's post-hoc analysis.
Adolescence and adulthood
From P30 onwards, the dorsal and ventral striatum were examined separately. There were no significant differences in [3H]DA uptake or K+-stimulated release at any age during late postnatal development (data not shown). For dorsal striatum (Table 2, Fig. 7 C & D), there were no significant age or sex differences in nicotine efficacy. Although there was a significant sex X age interaction in EC50 values (p<0.01, F(2,31)=6.55), further post-hoc analyses did not yield significant age or sex differences. (Table 2)
Figure 7.
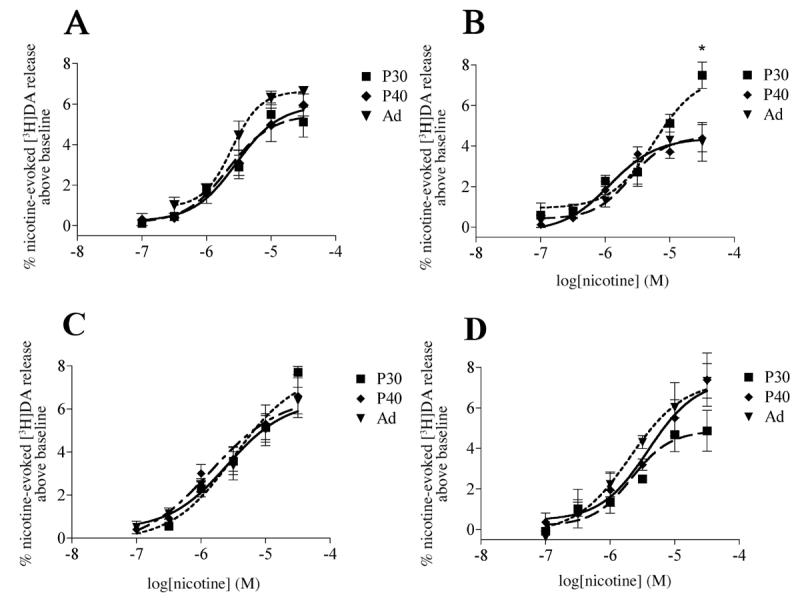
Dose-response curves for nicotine-stimulated [3H]DA release for P30, P40 and adult female (A) and male (B) ventral and female (C) and male (D) dorsal striatal slices. Release is significantly higher from P30 male ventral striatum when compared to either P40 or adult, whereas in the female ventral striaum, the release is similar at all three ages. There is no age-dependent difference in either sex in the dorsal striatum. Values are mean ± SEM from 3-6 independent experiments. *p<0.05, Dunnett's post-hoc analysis with adult as control.
In ventral striatum there were complex changes in the functional properties of nAChRs during the adolescent/adult period (Table 3). Significant sex X age interactions were found for both nicotine efficacy (p<0.05, F(2,19)=5.887) and EC50 values (p<0.0001, F(2,27)=20.82). Further post-hoc analyses indicated that significant age differences in both efficacy and potency occurred in males, but not females (Fig 7 A & B). In P30 males, maximal nicotine-stimulated [3H]DA release was significantly higher than at older ages (Table 3, Fig. 7B). As compared to adult males, nicotine was also significantly less potent at P30 and more potent at P40 (Table 3, Fig. 7B). (Table 3 & Figure 7)
Table 3.
Nicotine-stimulated [3H]DA release from ventral striatum. EC50s (95% confidence intervals) and maximum release (mean ± SEM) are from 3-6 independent experiments.
| Age | EC50 (μM) | Max. Release (%) | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| 30 | 5.75*** (3.4-9.7) |
2.18 (1.2-4.0) |
7.49 ± 0.65* | 5.49 ± 0.67 |
| 40 | 1.18* (0.5-2.8) |
2.9 (1.4-5.8) |
4.39 ± 0.75 | 5.95 ± 0.59 |
| Ad | 2.37 (1.0-6.0) |
2.46 (1.6-3.7) |
4.33 ± 0.65 | 6.64 ± 0.23 |
p<0.05
p<0.001
significantly different from adult of the same sex, Dunnett's post-hoc with adult as control.
DISCUSSION
The present study provides strong evidence for the presence of functional nAChRs on DA neurons even during the fetal period. As early as G15, only a couple of days following the appearance of TH within midbrain DA neurons (Specht et al., 1981, Voorn et al., 1988), nAChR subunit mRNAs and high affinity binding sites are present within these neurons, and functional receptors are detectable as early as G17-18. During the first three postnatal weeks, there is a gradual increase in both the efficacy and potency of nicotine, which parallels a decline in expression of some nAChR subunit mRNAs and a rise in expression of others.
Fetal period
Rat DA midbrain neurons are generated on days 12 -15 of gestation and migrate to the SNc and VTA shortly thereafter (Specht et al., 1981, Marchand and Poirier, 1983, Voorn et al., 1988). These neurons have been shown to differentiate biochemically when they are still morphologically immature (Specht et al., 1981). Although previous studies have shown the presence of nAChR subunit mRNAs and binding sites in ventral mesencephalon as early as G12 (Naeff et al., 1992, Zoli et al., 1995), we have now shown that nAChRs are expressed in immature DA neurons. Furthermore, migrating neurons may express nAChR subunit mRNAs prior to expression of TH mRNA. Whereas expression levels of most nAChR mRNAs are equivalent in fetal SNc and VTA, both α4 mRNA expression and [3H]nicotine binding are higher in SNc than VTA at G15. The reason for this difference in neurochemical differentiation of SNc and VTA is unknown but may reflect different birthdates and origins (Bayer et al., 1995). By G18, this regional difference in nAChR expression has disappeared and does not reemerge until later in postnatal development, when it is evident for α5, α6 and β3 mRNAs.
We have also shown the presence of functional nAChRs that regulate DA release from fetal striatal terminals. Although K+ depolarization induced significantly less [3H]DA release from fetal striatal slices than from animals aged two weeks and older, nicotine stimulated equivalent maximal levels of DA release at G17-18 as at P21. As compared to P21, however, nicotine potency was an order of magnitude lower in fetal brain. This finding, combined with our observation of changing developmental patterns of subunit expression and radioligand binding, suggests that fetal DA terminals express a nAChR with different properties from that of older animals.
It has been well documented that chronic prenatal nicotine exposure alters the development of DA neurons (Fung, 1988, Lichtensteiger et al., 1988, Navarro et al., 1988, Fung, 1989, Ribary and Lichtensteiger, 1989, Lichtensteiger and Schlumpf, 1993, Richardson and Tizabi, 1994, Muneoka et al., 1997). As suggested in the present study, one potential site of action for nicotine in producing these changes may be through direct action on nAChRs on immature DA neurons. Our present finding of functional nAChRs on fetal DA terminals is also consistent with recent evidence that point mutant mice with hypersensitive α4 subunit-containing nAChRs lose DA neurons during late fetal development (Labarca et al., 2001).
Early postnatal period
During the first three postnatal weeks, there are complex changes in the expression of nAChR subunit mRNAs in midbrain DA neurons. Whereas α3 and α4 subunit mRNAs decrease to lower adult levels, there is an emergence of α6 subunit expression. Other subunits which are present at high levels at G15, such as α5, also exhibit a secondary peak of expression during the postnatal period. Levels of α5, α6 and α7 mRNAs peak at P21 and then decline to lower adult levels. Regional differences in expression of α5, α6 and β3 mRNAs emerge during the postnatal period, with higher expression levels in SNc than VTA. Our previous quantitative analysis has shown that >90% of DA neurons within adult rat SNc and VTA express α5, α6 and β3 subunit mRNAs (Azam et al., 2002). Since high resolution microscopic analysis has shown that expression of these mRNAs at P21 is also largely restricted to DA neurons, the increased expression levels at this age, particularly within the SNc, appear to reflect increased levels of transcripts expressed per DA cell.
During the postnatal period there are also substantial changes in radioligand binding to nAChRs within SNc/VTA. In contrast to expression of some nAChR subunit mRNAs, no regional differences in radioligand binding were observed at any postnatal age. Whereas [125I]α-BTX binding sites reached adult levels by birth, and remained constant thereafter, there was a prolonged postnatal decline in [3H]NIC binding. Since we used only a single concentration of radioligand for these binding analyses, it is not clear whether this decline in binding reflects changes in binding site density or affinity. Our analysis was also restricted to DA cell body regions and did not include striatal terminal regions. However, a previous study of radioligand binding to α4β2-like nAChRs during the postnatal period has shown peak binding within striatum at P4 followed by a slow subsequent decline to adult levels (Aubert et al., 1996).
Our biochemical analysis of striatal slices has shown that DA uptake and depolarization-induced release do not mature until P14. Similarly, there is a slow postnatal maturation of nicotine-stimulated DA release, consistent with a developmental shift in subunit composition. By P21, the functional properties of nAChRs on dorsal striatal terminals have largely matured. Immediately after birth, there is a decline in nicotine's efficacy in both sexes, as indicated by the significantly lower maximal release, with a subsequent gradual increase to reach values similar to those seen in the fetal brain and adult. Since K+-stimulated [3H]DA release during the first postnatal week is also significantly lower than at later ages, it is possible that the lower nicotine-mediated release during this developmental period may result from a lower excitability of DA terminals. It has been demonstrated that prior to the end of the first postnatal week, there is a lack of impulse flow within the DA striatal efferents (Erinoff and Heller, 1978, Cheronis et al., 1979). It is therefore possible that, during the neonatal period, DA terminals have not acquired the necessary “machinery” for impulse-induced neurotransmitter release (e.g. voltage gated ion channels) which mediate nicotine's actions (Soliakov et al., 1995, Marshall et al., 1996, Prince et al., 1996, Kulak et al., 2001); however, this seems unlikely since K+-stimulated release was also significantly lower in fetal brain, whereas maximal levels of nicotine-stimulated transmitter release were similar to that obtained in mature brain. Thus, it seems likely that the early postnatal changes in nicotine actions reflect changes in nAChR properties.
There is also a complex developmental regulation of EC50 values in both sexes during the perinatal period. At birth, nicotine's potency increases in males whereas in females it remains as low as it was during late-gestation. Although in the present study, sex differences in EC50 values were not examined during gestational period, it is possible that the observed sex differences in nicotine's sensitivity at birth may also be present prenatally, with males exhibiting a higher sensitivity to nicotine. Gestational nicotine exposure has been shown to produce a sex-dependent alteration in DA systems, with many of the behavioral and biochemical effects occurring in males (Fung, 1988, Fung, 1989, Ribary and Lichtensteiger, 1989).
Adolescence/adulthood
Adolescence, as defined in rodent as the period between P28 and P42, is a period of active maturation of brain DA systems (Spear, 2000, Chambers et al., 2003). Over-production and pruning of DA receptors has been reported to occur in the striatum and frontal cortex of adolescent males, but not females (Andersen and Teicher, 2000). Such changes appear to reflect innate sex differences in the maturation of DA systems and not the acute effects of sex hormones (Andersen et al., 2002). Whereas we have found no further changes in the maturation of nAChR-mediated DA release in dorsal striatum after P21, there were complex changes in nAChR regulation of ventral striatal DA release in adolescent males but not females. Nicotine stimulated [3H]DA release from P30 male ventral striatal slices with a significantly higher efficacy but lower potency than adult animals. At P40, in contrast, nicotine was significantly more potent than in adult although the maximal release had reached adult levels.
DA release within the ventral striatum is believed to underlie the addictive/reinforcing properties of nicotine (Imperato et al., 1986, Corrigall et al., 1992, Nisell et al., 1995). Therefore, the present data suggest that the observed higher maximum nicotine-stimulated [3H]DA release at P30 and higher sensitivity at P40 may render this drug highly rewarding and reinforcing in males, during a developmental period that corresponds to adolescence (Spear, 2000). In humans, adolescence is a period of high risk for initiation and maintenance of cigarette use, as more than 80% of adult smokers have started smoking as adolescents. The age of smoking initiation also seems to be of importance, as a younger age of smoking initiation was associated with heavier smoking at an older age (Everett et al., 1999). Moreover, it has been found that males are more likely than females to initiate smoking at an early age (Robinson and Klesges, 1997, Everett et al., 1999) with this difference disappearing after puberty (Everett et al., 1999). If the results from nicotine-mediated [3H]DA release in rats can be extended to humans, it suggests that human adolescence may be a period of high stimulatory effects of nicotine on the male DA system. A recent study has indeed shown that pretreatment with nicotine during the periadolescent (30-40 days old), but not postadolescent, male rats increases nicotine self-administration in adult subjects (Adriani et al., 2003)
The sex differences that were observed in the present study all occur during the prepubertal period, before the surge of gonadal hormones which have been shown to allosterically modulate nAChRs and inhibit nicotine-mediated [3H]DA release (Bertrand et al., 1991, Valera et al., 1992, Bullock et al., 1997, Paradiso et al., 2000). Although it is possible that these functional sex differences may reflect inherent sex differences within midbrain DAergic neurons (Engele et al., 1989, Beyer et al., 1991, Reisert and Pilgrim, 1991, Ovtscharoff et al., 1992), this seems unlikely since there were no significant sex differences in [3H]DA uptake or K+-stimulated transmitter release. In view of the lack of substantial sex differences in the developmental profile of nAChR mRNAs and protein that we have observed, these differences may reflect sex differences in post-translational modification of nAChR subunits which may affect receptor composition.
Physiological significance of nAChR composition during development
Functional neurotransmitter release studies in adult rat striatal synaptosomes have suggested the involvement of α6β2*1 (Azam and McIntosh, 2005) and α4β2* (Sharples et al., 2000) nAChRs in the modulation of [3H]DA release. In addition, immunoprecipitation studies have indicated the presence of complex nAChR subtypes on adult rat striatal DAergic terminals: α4β2, α4α5β2, α4α6β2β3 and α6β2β3 (Zoli et al., 2002, Champtiaux et al., 2003). Although no α3 and α7 subunits appear to be present on adult striatal DAergic terminals, these subunits may participate in formation of nAChRs on the DAergic cell bodies located within the SNc/VTA (Wooltorton et al., 2003, Matsubayashi et al., 2004a, Matsubayashi et al., 2004b, Emmett and Greenfield, 2005).
Our present data suggest that nAChR subunit composition may change substantially with age. Furthermore, there may be significant differences in the subunit composition of nAChRs within DA neurons of the SNc and VTA, and their respective terminal regions, during later postnatal development. The anatomical data from the present study suggest that α4-containing nAChRs may be more important in modulation of striatal DAergic neurotransmission during the gestational period, whereas in mature animals α6-containing nAChRs also become important. The developmental profile of the α5 subunit mRNA suggests an important role for this subunit both prenatally and at later developmental stages. Interestingly, the maximum DA release profile during development matches closely with the developmental profile of the α5 mRNA. This subunit has been shown to enhance the conductance and Ca2+ permeability when incorporated in nAChRs (Gerzanich et al., 1998). It is possible that the higher maximum release during prenatal and later developmental ages is due to the participation of the α5 subunit in the formation of nAChRs. Such changes in subunit composition may be important in fine-tuning the sensitivity and/or kinetics of receptors to optimize neuronal excitability in face of changes in endogenous neurotransmitter levels. Further detailed pharmacological studies, including use of subtype selective α-conotoxins, such as α-conotoxins MII (and analogs) and PIA (Dowell et al., 2003, McIntosh et al., 2004), will be required to evaluate the subunit composition of nAChRs on DA terminals during development.
Acknowledgements
This work was supported by NIH grant DA10612 and a Predoctoral Research Fellowship (L.A.) from the UC Tobacco Related Disease Research Program.
Abbreviations
- nAChR
nicotinic acetylcholine receptor
- SNc
substantia nigra
- VTA
ventral tegmental area
- NIC
nicotine
- α-BTX
α-bungarotoxin
- DA
dopamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Asterisk indicates presence of additional subunits.
Section Editor
Developmental: Dr. John L. R. Rubenstein, Nina Ireland Distinguished Professor in Child Psychiatry, Nina Ireland Laboratory of Developmental Neurobiology, Center for Neurobiology and Psychiatry, Genetics, Development and Behavioral Sciences Building, 1550 4th Street, 2nd Floor South, Room GD 284C, University of California at San Francisco, San Francisco, CA 94143-2611.
Literature Cited
- 1.Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajarem JS, Ahmad M. Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol Biochem Behav. 1998;59:313–318. doi: 10.1016/s0091-3057(97)00408-5. [DOI] [PubMed] [Google Scholar]
- 3.Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 4.Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–691. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- 5.Aubert I, Cecyre D, Gauthier S, Quirion R. Comparative ontogenic profile of cholinergic markers, including nicotinic and muscarinic receptors, in the rat brain. J Comp Neurol. 1996;369:31–55. doi: 10.1002/(SICI)1096-9861(19960520)369:1<31::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Azam L, McIntosh JM. Effect of novel alpha-conotoxins on nicotine-stimulated [3H]dopamine release from rat striatal synaptosomes. J Pharmacol Exp Ther. 2005;312:231–237. doi: 10.1124/jpet.104.071456. [DOI] [PubMed] [Google Scholar]
- 7.Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- 8.Bayer SA, Wills KV, Triarhou LC, Ghetti B. Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp Brain Res. 1995;105:191–199. doi: 10.1007/BF00240955. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand D, Valera S, Bertrand S, Ballivet M, Rungger D. Steroids inhibit nicotinic acetylcholine receptors. Neuroreport. 1991;2:277–280. doi: 10.1097/00001756-199105000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Beyer C, Pilgrim C, Reisert I. Dopamine content and metabolism in mesencephalic and diencephalic cell cultures: sex differences and effects of sex steroids. J Neurosci. 1991;11:1325–1333. doi: 10.1523/JNEUROSCI.11-05-01325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bullock AE, Clark AL, Grady SR, Robinson SF, Slobe BS, Marks MJ, Collins AC. Neurosteroids modulate nicotinic receptor function in mouse striatal and thalamic synaptosomes. J Neurochem. 1997;68:2412–2423. doi: 10.1046/j.1471-4159.1997.68062412.x. [DOI] [PubMed] [Google Scholar]
- 12.Castellanos FX. Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clin Pediatr (Phila) 1997;36:381–393. doi: 10.1177/000992289703600702. [DOI] [PubMed] [Google Scholar]
- 13.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charpantier E, Barneoud P, Moser P, Besnard F, Sgard F. Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport. 1998;9:3097–3101. doi: 10.1097/00001756-199809140-00033. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Patrick JW. The alpha-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the alpha7 subunit. J Biol Chem. 1997;272:24024–24029. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- 17.Cheronis JC, Erinoff L, Heller A, Hoffmann PC. Pharmacological analysis of the functional ontogeny of the nigrostriatal dopaminergic neurons. Brain Res. 1979;169:545–560. doi: 10.1016/0006-8993(79)90403-7. [DOI] [PubMed] [Google Scholar]
- 18.Clarke PB, Reuben M. Release of [3H]-noradrenaline from rat hippocampal synaptosomes by nicotine: mediation by different nicotinic receptor subtypes from striatal [3H]-dopamine release. Br J Pharmacol. 1996;117:595–606. doi: 10.1111/j.1476-5381.1996.tb15232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- 20.Cox KH, DeLeon DV, Angerer LM, Angerer RC. Detection of mRNAs in sea urchin embryos by in situ using asymmetric RNA probes. Dev Biol. 1984;101:485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- 21.Dowell C, Olivera BM, Garrett JE, Staheli ST, Watkins M, Kuryatov A, Yoshikami D, Lindstrom JM, McIntosh JM. Alpha-conotoxin PIA is selective for alpha6 subunit-containing nicotinic acetylcholine receptors. J Neurosci. 2003;23:8445–8452. doi: 10.1523/JNEUROSCI.23-24-08445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drisdel RC, Green WN. Neuronal alpha-bungarotoxin receptors are alpha7 subunit homomers. J Neurosci. 2000;20:133–139. doi: 10.1523/JNEUROSCI.20-01-00133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott KJ, Jones JM, Sacaan AI, Lloyd GK, Corey-Naeve J. 6-hydroxydopamine lesion of rat nigrostriatal dopaminergic neurons differentially affects nicotinic acetylcholine receptor subunit mRNA expression. J Mol Neurosci. 1998;10:251–260. doi: 10.1007/BF02761778. [DOI] [PubMed] [Google Scholar]
- 24.Emmett SR, Greenfield SA. Correlation between dopaminergic neurons, acetylcholinesterase and nicotinic acetylcholine receptors containing the alpha3- or alpha5-subunit in the rat substantia nigra. J Chem Neuroanat. 2005;30:34–44. doi: 10.1016/j.jchemneu.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Engele J, Pilgrim C, Reisert I. Sexual differentiation of mesencephalic neurons in vitro: effects of sex and gonadal hormones. Int J Dev Neurosci. 1989;7:603–611. doi: 10.1016/0736-5748(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 26.Erinoff L, Heller A. Functional ontogeny of nigrostriatal neurons. Brain Res. 1978;142:566–569. doi: 10.1016/0006-8993(78)90919-8. [DOI] [PubMed] [Google Scholar]
- 27.Everett SA, Warren CW, Sharp D, Kann L, Husten CG, Crossett LS. Initiation of cigarette smoking and subsequent smoking behavior among U.S. high school students. Prev Med. 1999;29:327–333. doi: 10.1006/pmed.1999.0560. [DOI] [PubMed] [Google Scholar]
- 28.Fung YK. Postnatal behavioural effects of maternal nicotine exposure in rats. J Pharm Pharmacol. 1988;40:870–872. doi: 10.1111/j.2042-7158.1988.tb06290.x. [DOI] [PubMed] [Google Scholar]
- 29.Fung YK. Postnatal effects of maternal nicotine exposure on the striatal dopaminergic system in rats. J Pharm Pharmacol. 1989;41:576–578. doi: 10.1111/j.2042-7158.1989.tb06533.x. [DOI] [PubMed] [Google Scholar]
- 30.Fung YK, Lau YS. Effects of prenatal nicotine exposure on rat striatal dopaminergic and nicotinic systems. Pharmacol Biochem Behav. 1989;33:1–6. doi: 10.1016/0091-3057(89)90419-x. [DOI] [PubMed] [Google Scholar]
- 31.Gerzanich V, Wang F, Kuryatov A, Lindstrom J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- 32.Grady S, Marks MJ, Wonnacott S, Collins AC. Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992;59:848–856. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- 33.Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- 34.Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulak JM, McIntosh JM, Yoshikami D, Olivera BM. Nicotine-evoked transmitter release from synaptosomes: functional association of specific presynaptic acetylcholine receptors and voltage-gated calcium channels. J Neurochem. 2001;77:1581–1589. doi: 10.1046/j.1471-4159.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 36.Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, Nashmi R, Kofuji P, Dang H, Shi W, Fidan M, Khakh BS, Chen Z, Bowers BJ, Boulter J, Wehner JM, Lester HA. Point mutant mice with hypersensitive alpha 4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci U S A. 2001;98:2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lichtensteiger W, Ribary U, Schlumpf M, Odermatt B, Widmer HR. Prenatal adverse effects of nicotine on the developing brain. Prog Brain Res. 1988;73:137–157. doi: 10.1016/S0079-6123(08)60502-6. [DOI] [PubMed] [Google Scholar]
- 38.Lichtensteiger W, Schlumpf M. Prenatal nicotine exposure: biochemical and neuroendocrine bases of behavioral dysfunction. Dev Brain Dysfunction. 1993;6:279–304. [Google Scholar]
- 39.Marchand R, Poirier LJ. Isthmic origin of neurons of the rat substantia nigra. Neuroscience. 1983;9:373–381. doi: 10.1016/0306-4522(83)90300-7. [DOI] [PubMed] [Google Scholar]
- 40.Marshall D, Soliakov L, Redfern P, Wonnacott S. Tetrodotoxin-sensitivity of nicotine-evoked dopamine release from rat striatum. Neuropharmacology. 1996;35:1531–1536. doi: 10.1016/s0028-3908(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 41.Matsubayashi H, Amano T, Seki T, Sasa M, Sakai N. Postsynaptic alpha 4 beta 2 and alpha 7 type nicotinic acetylcholine receptors contribute to the local and endogenous acetylcholine-mediated synaptic transmissions in nigral dopaminergic neurons. Brain Res. 2004a;1005:1–8. doi: 10.1016/j.brainres.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 42.Matsubayashi H, Inoue A, Amano T, Seki T, Nakata Y, Sasa M, Sakai N. Involvement of alpha7- and alpha4beta2-type postsynaptic nicotinic acetylcholine receptors in nicotine-induced excitation of dopaminergic neurons in the substantia nigra: a patch clamp and single-cell PCR study using acutely dissociated nigral neurons. Brain Res Mol Brain Res. 2004b;129:1–7. doi: 10.1016/j.molbrainres.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 43.McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, Garrett JE, Marks MJ, Whiteaker P. Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol Pharmacol. 2004;65:944–952. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- 44.Millberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am. J. Psychiat. 1996;153:1138–1142. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- 45.Miller JA, Zahniser NR. The use of 14C-labeled tissue paste standards for the calibration of 125I-labeled ligands in quantitative autoradiography. Neurosci Lett. 1987;81:345–350. doi: 10.1016/0304-3940(87)90408-3. [DOI] [PubMed] [Google Scholar]
- 46.Muneoka K, Ogawa T, Kamei K, Muraoka S, Tomiyoshi R, Mimura Y, Kato H, Suzuki MR, Takigawa M. Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: involvement of route of drug administrations. Brain Res Dev Brain Res. 1997;102:117–126. doi: 10.1016/s0165-3806(97)00092-8. [DOI] [PubMed] [Google Scholar]
- 47.Naeff B, Schlumpf M, Lichtensteiger W. Pre- and postnatal development of high-affinity [3H]nicotine binding sites in rat brain regions: an autoradiographic study. Brain Res Dev Brain Res. 1992;68:163–174. doi: 10.1016/0165-3806(92)90058-5. [DOI] [PubMed] [Google Scholar]
- 48.Navarro HA, Seidler FJ, Whitmore WL, Slotkin TA. Prenatal exposure to nicotine via maternal infusions: effects on development of catecholamine systems. J Pharmacol Exp Ther. 1988;244:940–944. [PubMed] [Google Scholar]
- 49.Nisell M, Nomikos GG, Svensson TH. Nicotine dependence, midbrain dopamine systems and psychiatric disorders. Pharmacol Toxicol. 1995;76:157–162. doi: 10.1111/j.1600-0773.1995.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 50.Ovtscharoff W, Eusterschulte B, Zienecker R, Reisert I, Pilgrim C. Sex differences in densities of dopaminergic fibers and GABAergic neurons in the prenatal rat striatum. J Comp Neurol. 1992;323:299–304. doi: 10.1002/cne.903230212. [DOI] [PubMed] [Google Scholar]
- 51.Paradiso K, Sabey K, Evers AS, Zorumski CF, Covey DF, Steinbach JH. Steroid inhibition of rat neuronal nicotinic alpha4beta2 receptors expressed in HEK 293 cells. Mol Pharmacol. 2000;58:341–351. doi: 10.1124/mol.58.2.341. [DOI] [PubMed] [Google Scholar]
- 52.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1986. [Google Scholar]
- 53.Perry DC, Kellar KJ. [3H]epibatidine labels nicotinic receptors in rat brain: an autoradiographic study. J Pharmacol Exp Ther. 1995;275:1030–1034. [PubMed] [Google Scholar]
- 54.Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 55.Pliszka SR, McCracken JT, Maas JW. Catecholamines in attention-deficit hyperactivity disorder: current perspectives. J Am Acad Child Adolesc Psychiatry. 1996;35:264–272. doi: 10.1097/00004583-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Prince RJ, Fernandes KG, Gregory JC, Martyn ID, Lippiello PM. Modulation of nicotine-evoked [3H]dopamine release from rat striatal synaptosomes by voltage-sensitive calcium channel ligands. Biochem Pharmacol. 1996;52:613–618. doi: 10.1016/0006-2952(96)00313-9. [DOI] [PubMed] [Google Scholar]
- 57.Rapier C, Lunt GG, Wonnacott S. Nicotinic modulation of [3H]dopamine release from striatal synaptosomes: pharmacological characterisation. J Neurochem. 1990;54:937–945. doi: 10.1111/j.1471-4159.1990.tb02341.x. [DOI] [PubMed] [Google Scholar]
- 58.Reisert I, Pilgrim C. Sexual differentiation of monoaminergic neurons--genetic or epigenetic? Trends Neurosci. 1991;14:468–473. doi: 10.1016/0166-2236(91)90047-x. [DOI] [PubMed] [Google Scholar]
- 59.Ribary U, Lichtensteiger W. Effects of acute and chronic prenatal nicotine treatment on central catecholamine systems of male and female rat fetuses and offspring. J Pharmacol Exp Ther. 1989;248:786–792. [PubMed] [Google Scholar]
- 60.Richardson SA, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacol Biochem Behav. 1994;47:331–337. doi: 10.1016/0091-3057(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 61.Robinson LA, Klesges RC. Ethnic and gender differences in risk factors for smoking onset. Health Psychol. 1997;16:499–505. doi: 10.1037//0278-6133.16.6.499. [DOI] [PubMed] [Google Scholar]
- 62.Schlumpf M, Gahwiler M, Ribary U, Lichtensteiger W. A new device for monitoring early motor development: prenatal nicotine-induced changes. Pharmacol Biochem Behav. 1988;30:199–203. doi: 10.1016/0091-3057(88)90444-3. [DOI] [PubMed] [Google Scholar]
- 63.Sharples CG, Kaiser S, Soliakov L, Marks MJ, Collins AC, Washburn M, Wright E, Spencer JA, Gallagher T, Whiteaker P, Wonnacott S. UB-165: a novel nicotinic agonist with subtype selectivity implicates the alpha4beta2* subtype in the modulation of dopamine release from rat striatal synaptosomes. J Neurosci. 2000;20:2783–2791. doi: 10.1523/JNEUROSCI.20-08-02783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabeled single-stranded RNA probes. J. Histotechnol. 1989;12:169–181. [Google Scholar]
- 65.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- 66.Soliakov L, Gallagher T, Wonnacott S. Anatoxin-a-evoked [3H]dopamine release from rat striatal synaptosomes. Neuropharmacology. 1995;34:1535–1541. doi: 10.1016/0028-3908(95)00122-m. [DOI] [PubMed] [Google Scholar]
- 67.Sorenson EM, Shiroyama T, Kitai ST. Postsynaptic nicotinic receptors on dopaminergic neurons in the substantia nigra pars compacta of the rat. Neuroscience. 1998;87:659–673. doi: 10.1016/s0306-4522(98)00064-5. [DOI] [PubMed] [Google Scholar]
- 68.Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- 69.Specht LA, Pickel VM, Joh TH, Reis DJ. Fine structure of the nigrostriatal anlage in fetal rat brain by immunocytochemical localization of tyrosine hydroxylase. Brain Res. 1981;218:49–65. doi: 10.1016/0006-8993(81)90988-4. [DOI] [PubMed] [Google Scholar]
- 70.Tizabi Y, Popke EJ, Rahman MA, Nespor SM, Grunberg NE. Hyperactivity induced by prenatal nicotine exposure is associated with an increase in cortical nicotinic receptors. Pharmacol Biochem Behav. 1997;58:141–146. doi: 10.1016/s0091-3057(96)00461-3. [DOI] [PubMed] [Google Scholar]
- 71.Valera S, Ballivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1992;89:9949–9953. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voorn P, Kalsbeek A, Jorritsma-Byham B, Groenewegen HJ. The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience. 1988;25:857–887. doi: 10.1016/0306-4522(88)90041-3. [DOI] [PubMed] [Google Scholar]
- 73.Winzer-Serhan UH, Leslie FM. Codistribution of nicotinic acetylcholine receptor subunit alpha3 and beta4 mRNAs during rat brain development. J Comp Neurol. 1997;386:540–554. doi: 10.1002/(sici)1096-9861(19971006)386:4<540::aid-cne2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 74.Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- 75.Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin R, French ED. A comparison of the effects of nicotine on dopamine and non-dopamine neurons in the rat ventral tegmental area: an in vitro electrophysiological study. Brain Res Bull. 2000;51:507–514. doi: 10.1016/s0361-9230(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 77.Zoli M, Le Novere N, Hill JA, Jr., Changeux JP. Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. J Neurosci. 1995;15:1912–1939. doi: 10.1523/JNEUROSCI.15-03-01912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


