Abstract
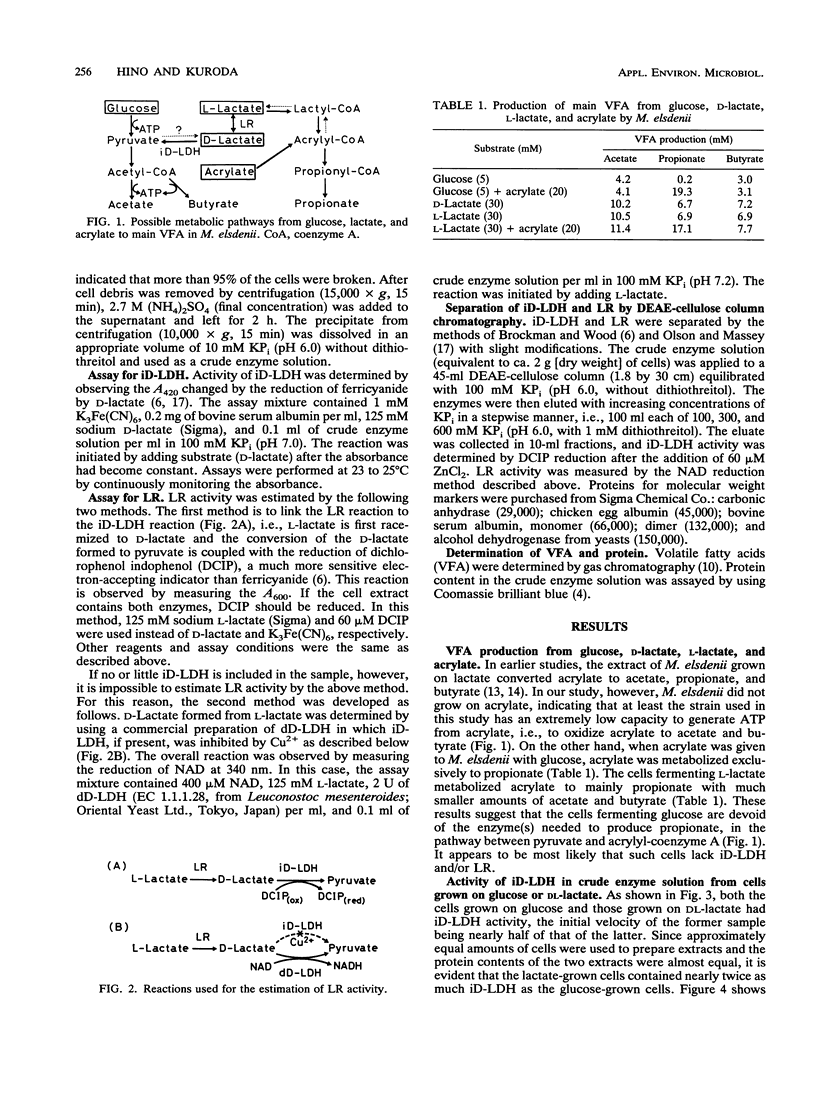
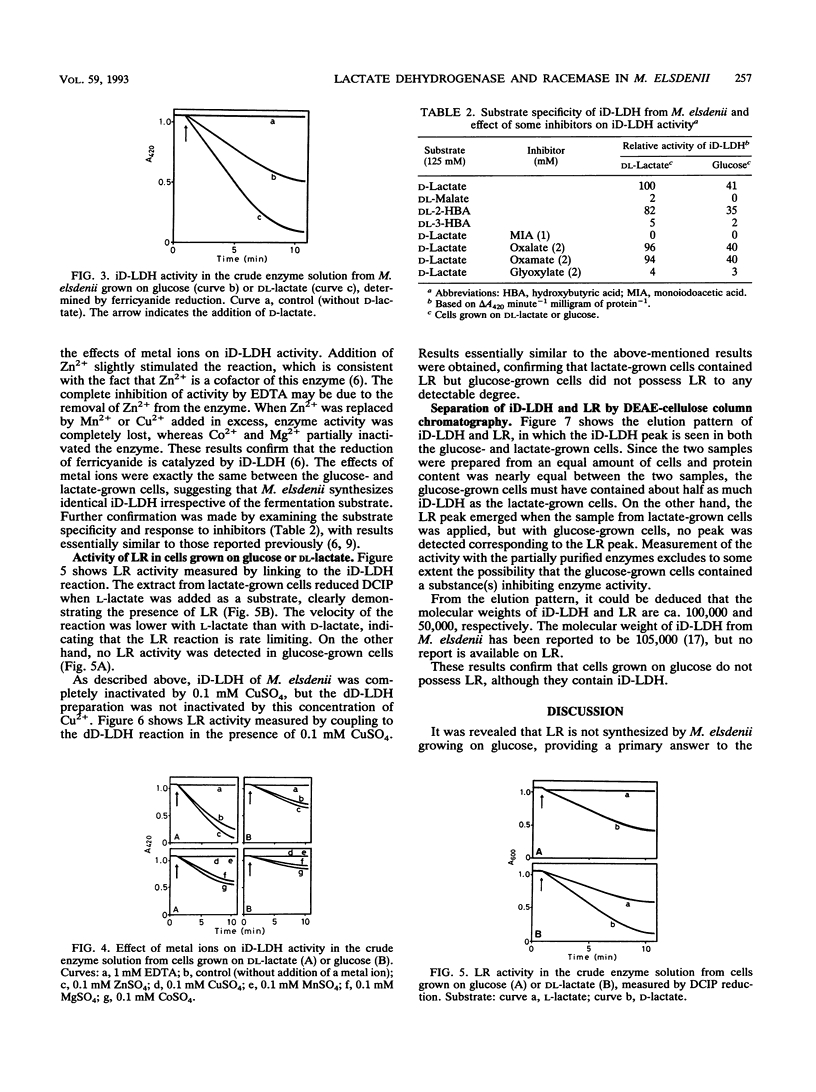
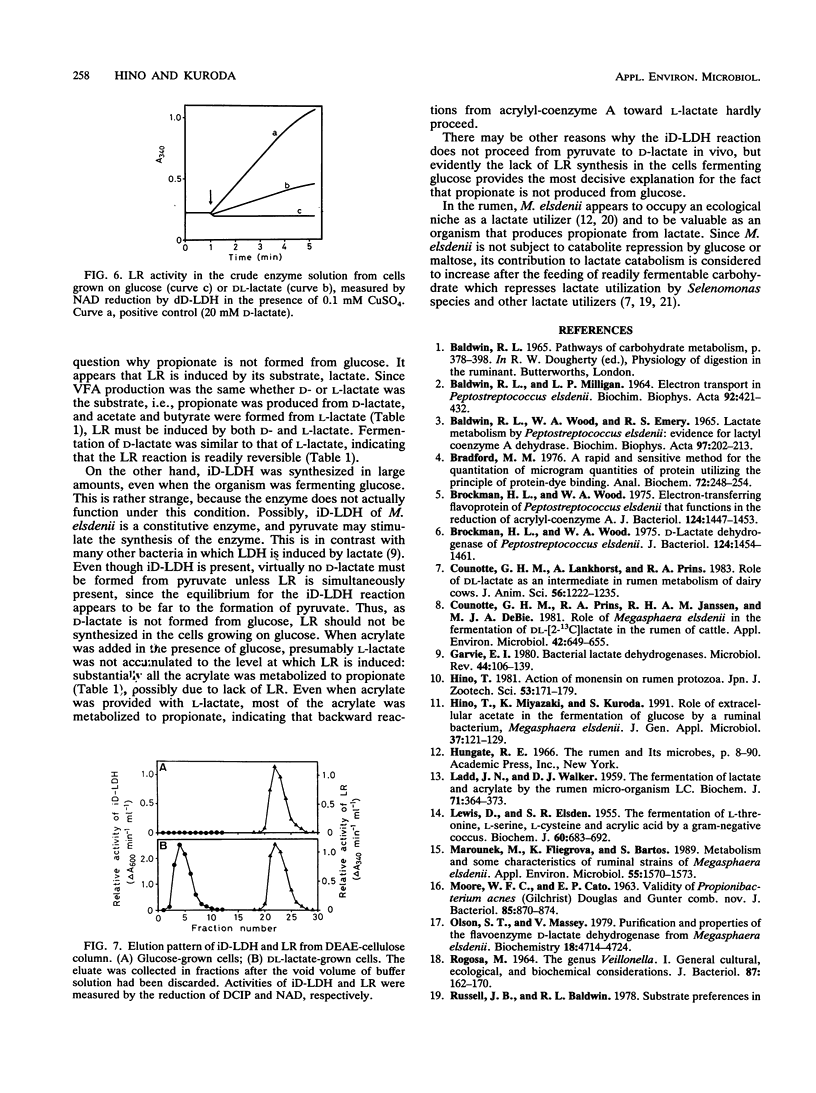
Activity of D-lactate dehydrogenase (D-LDH) was shown not only in cell extracts from Megasphaera elsdenii grown on DL-lactate, but also in cell extracts from glucose-grown cells, although glucose-grown cells contained approximately half as much D-LDH as DL-lactate-grown cells. This indicates that the D-LDH of M. elsdenii is a constitutive enzyme. However, lactate racemase (LR) activity was present in DL-lactate-grown cells, but was not detected in glucose-grown cells, suggesting that LR is induced by lactate. Acetate, propionate, and butyrate were produced similarly from both D- and L-lactate, indicating that LR can be induced by both D- and L-lactate. These results suggest that the primary reason for the inability of M. elsdenii to produce propionate from glucose is that cells fermenting glucose do not synthesize LR, which is induced by lactate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDWIN R. L., MILLIGAN L. P. ELECTRON TRANSPORT IN PEPTOSTREPTOCOCCUS ELSDENII. Biochim Biophys Acta. 1964 Dec 23;92:421–432. doi: 10.1016/0926-6569(64)90001-x. [DOI] [PubMed] [Google Scholar]
- BALDWIN R. L., WOOD W. A., EMERY R. S. LACTATE METABOLISM BY PEPTOSTREPTOCOCCUS ELSDENII: EVIDENCE FOR LACTYL COENZYME A DEHYDRASE. Biochim Biophys Acta. 1965 Feb 15;97:202–213. doi: 10.1016/0304-4165(65)90084-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brockman H. L., Wood W. A. D-Lactate dehydrogenase of Peptostreptococcus elsdenii. J Bacteriol. 1975 Dec;124(3):1454–1461. doi: 10.1128/jb.124.3.1454-1461.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman H. L., Wood W. A. Electron-transferring flavoprotein of Peptostreptococcus elsdenii that functions in the reduction of acrylyl-coenzyme A. J Bacteriol. 1975 Dec;124(3):1447–1453. doi: 10.1128/jb.124.3.1447-1453.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte G. H., Lankhorst A., Prins R. A. Role of DL-lactic acid as an intermediate in rumen metabolism of dairy cows. J Anim Sci. 1983 May;56(5):1222–1235. doi: 10.2527/jas1983.5651222x. [DOI] [PubMed] [Google Scholar]
- Counotte G. H., Prins R. A., Janssen R. H., Debie M. J. Role of Megasphaera elsdenii in the Fermentation of dl-[2-C]lactate in the Rumen of Dairy Cattle. Appl Environ Microbiol. 1981 Oct;42(4):649–655. doi: 10.1128/aem.42.4.649-655.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LADD J. N., WALKER D. J. The fermentation of lactate and acrylate by the rumen micro-organism LC. Biochem J. 1959 Feb;71(2):364–373. doi: 10.1042/bj0710364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS D., ELSDEN S. R. The fermentation of L-threonine, L-serine, L-cysteine and acrylic acid by a gram-negative coccus. Biochem J. 1955 Aug;60(4):683–692. doi: 10.1042/bj0600683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE W. E., CATO E. P. VALIDITY OF PROPIONIBACTERIUM ACNES (GILCHRIST) DOUGLAS AND GUNTER COMB. NOV. J Bacteriol. 1963 Apr;85:870–874. doi: 10.1128/jb.85.4.870-874.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marounek M., Fliegrova K., Bartos S. Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii. Appl Environ Microbiol. 1989 Jun;55(6):1570–1573. doi: 10.1128/aem.55.6.1570-1573.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson S. T., Massey V. Purification and properties of the flavoenzyme D-lactate dehydrogenase from Megasphaera elsdenii. Biochemistry. 1979 Oct 16;18(21):4714–4724. doi: 10.1021/bi00588a036. [DOI] [PubMed] [Google Scholar]
- ROGOSA M. THE GENUS VEILLONELLA. I. GENERAL CULTURAL, ECOLOGICAL, AND BIOCHEMICAL CONSIDERATIONS. J Bacteriol. 1964 Jan;87:162–170. doi: 10.1128/jb.87.1.162-170.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Substrate preferences in rumen bacteria: evidence of catabolite regulatory mechanisms. Appl Environ Microbiol. 1978 Aug;36(2):319–329. doi: 10.1128/aem.36.2.319-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Cotta M. A., Dombrowski D. B. Rumen Bacterial Competition in Continuous Culture: Streptococcus bovis Versus Megasphaera elsdenii. Appl Environ Microbiol. 1981 Jun;41(6):1394–1399. doi: 10.1128/aem.41.6.1394-1399.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung K. K., Wood W. A. Purification, new assay, and properties of coenzyme A transferase from Peptostreptococcus elsdenii. J Bacteriol. 1975 Dec;124(3):1462–1474. doi: 10.1128/jb.124.3.1462-1474.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



