Abstract
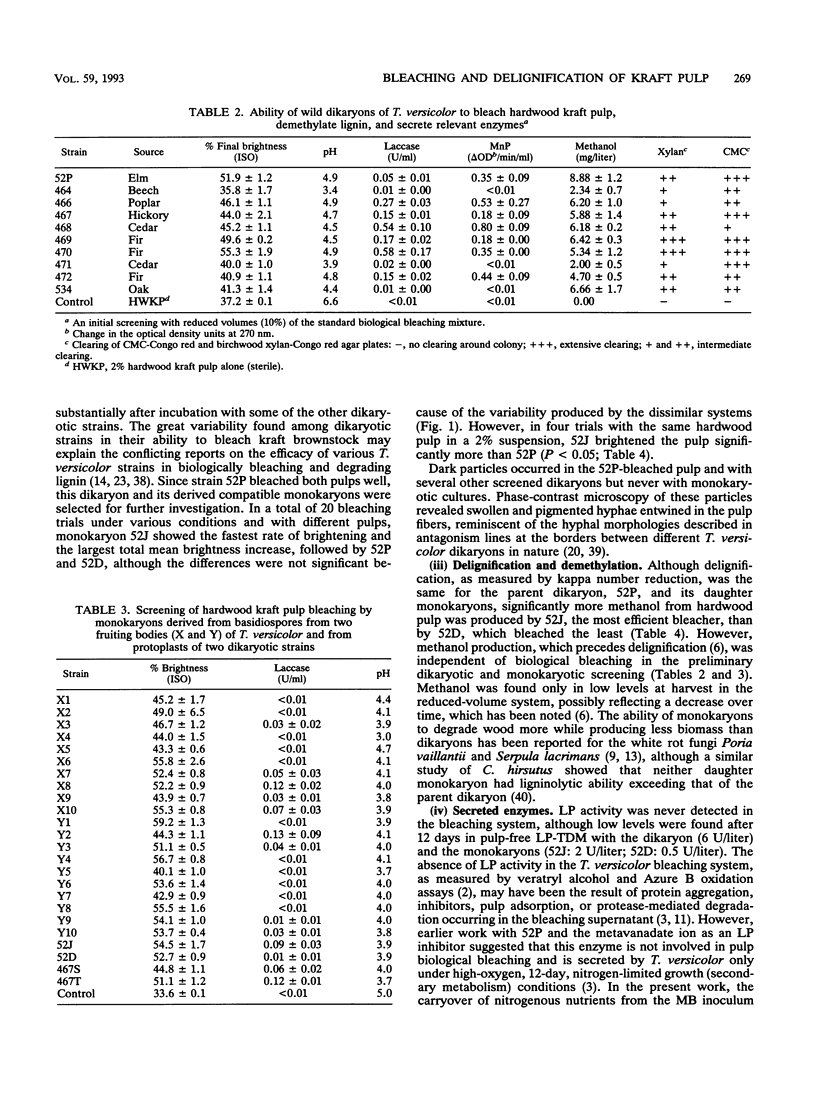
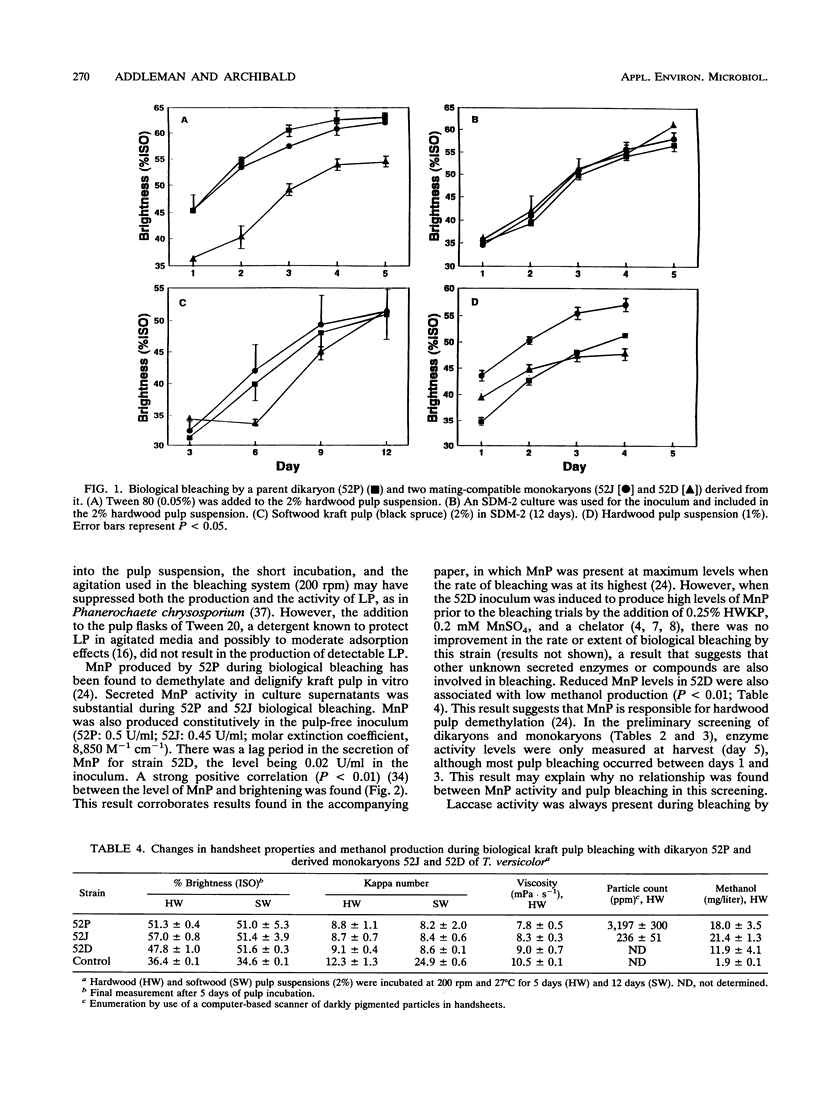
The ability of 10 dikaryotic and 20 monokaryotic strains of Trametes (Coriolus) versicolor to bleach and delignify hardwood and softwood kraft pulps was assessed. A dikaryon (52P) and two of its mating-compatible monokaryons (52J and 52D) derived via protoplasting were compared. All three regularly bleached hardwood kraft pulp more than 20 brightness points (International Standards Organization) in 5 days and softwood kraft pulp the same amount in 12 days. Delignification (kappa number reduction) by the dikaryon and the monokaryons was similar, but the growth of the monokaryons was slower. Insoluble dark pigments were commonly found in the mycelium, medium, and pulp of the dikaryon only. Laccase and manganese peroxidase (MnP) but not lignin peroxidase activities were secreted during bleaching by all three strains. Their laccase and MnP isozyme patterns were compared on native gels. No segregation of isozyme bands between the monokaryons was found. Hardwood kraft pulp appeared to adsorb several laccase isozyme bands. One MnP isozyme (pI, 3.2) was secreted in the presence of pulp by all three strains, but a second (pI, 4.9) was produced only by 52P. A lower level of soluble MnP activity in one monokaryon (52D) was associated with reduced bleaching ability and a lower level of methanol production. Since monokaryon 52J bleached pulp better than its parent dikaryon 52P, especially per unit of biomass, this genetically simpler monokaryon will be the preferred subject for further genetic manipulation and improvement of fungal pulp biological bleaching.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S. A new assay for lignin-type peroxidases employing the dye azure B. Appl Environ Microbiol. 1992 Sep;58(9):3110–3116. doi: 10.1128/aem.58.9.3110-3116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S. Lignin Peroxidase Activity Is Not Important in Biological Bleaching and Delignification of Unbleached Kraft Pulp by Trametes versicolor. Appl Environ Microbiol. 1992 Sep;58(9):3101–3109. doi: 10.1128/aem.58.9.3101-3109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbonnais R., Paice M. G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990 Jul 2;267(1):99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- Brown J. A., Alic M., Gold M. H. Manganese peroxidase gene transcription in Phanerochaete chrysosporium: activation by manganese. J Bacteriol. 1991 Jul;173(13):4101–4106. doi: 10.1128/jb.173.13.4101-4106.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. A., Glenn J. K., Gold M. H. Manganese regulates expression of manganese peroxidase by Phanerochaete chrysosporium. J Bacteriol. 1990 Jun;172(6):3125–3130. doi: 10.1128/jb.172.6.3125-3130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoretz C. G., Dass S. B., Reddy C. A., Grethlein H. E. Protease-mediated degradation of lignin peroxidase in liquid cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Nov;56(11):3429–3434. doi: 10.1128/aem.56.11.3429-3434.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger A., Croan S., Kirk T. K. Production of Ligninases and Degradation of Lignin in Agitated Submerged Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Nov;50(5):1274–1278. doi: 10.1128/aem.50.5.1274-1278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y., Tsukuda Y., Kawai Y., Tsukamoto A., Sugiura J., Sakaino M., Kita Y. Cloning, sequence analysis, and expression of ligninolytic phenoloxidase genes of the white-rot basidiomycete Coriolus hirsutus. J Biol Chem. 1990 Sep 5;265(25):15224–15230. [PubMed] [Google Scholar]
- Mandels M., Andreotti R., Roche C. Measurement of saccharifying cellulase. Biotechnol Bioeng Symp. 1976;(6):21–33. [PubMed] [Google Scholar]
- Paice M. G., Reid I. D., Bourbonnais R., Archibald F. S., Jurasek L. Manganese Peroxidase, Produced by Trametes versicolor during Pulp Bleaching, Demethylates and Delignifies Kraft Pulp. Appl Environ Microbiol. 1993 Jan;59(1):260–265. doi: 10.1128/aem.59.1.260-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J., Jeffries T. W. Mineralization of C-Ring-Labeled Synthetic Lignin Correlates with the Production of Lignin Peroxidase, not of Manganese Peroxidase or Laccase. Appl Environ Microbiol. 1990 Jun;56(6):1806–1812. doi: 10.1128/aem.56.6.1806-1812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périé F. H., Gold M. H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl Environ Microbiol. 1991 Aug;57(8):2240–2245. doi: 10.1128/aem.57.8.2240-2245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatadri R., Irvine R. L. Effect of Agitation on Ligninase Activity and Ligninase Production by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Sep;56(9):2684–2691. doi: 10.1128/aem.56.9.2684-2691.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]




