Abstract
Cell directional orientation or shape polarization is the first cellular step in neutrophil locomotion. To better understand how chemoattractants interact with cells, we studied neutrophil polarization (or shape changes) during exposure to a temporally decreasing chemoattractant signal of N-formyl-methionyl-leucyl-phenylalanine (FMLP) in the absence of a spatial concentration gradient. To accomplish this objective, we used a manifold of differing FMLP concentrations attached to a stopped-flow microscope chamber. Spatial gradients of a fluorescent chemotactic peptide could not be detected in the chamber by using microfluorometry. When FMLP was injected at continually increasing concentrations at 10-s intervals, the shape and relative direction of the neutrophil persisted. However, when temporally decreasing FMLP concentrations were injected, ≈80% of the cells changed their direction with 44% of the total cells swinging about to 180° ± 15°. Most of these directional changes involved dissolution of both the lamellipodium and uropod and reformation of these structures 180° from their original positions. This research suggests that neutrophils reverse their morphological polarity when exposed to temporally decreasing ligand concentrations by “remembering” their ligand exposure history and relative direction.
Keywords: cell motility, cell polarization, shape change, signaling, chemokinesis
Neutrophils participate in inflammatory responses, such as host resistance to infectious disease, and in deleterious host inflammatory reactions, including arthritis, septic shock, and ischemia/reperfusion injury of tissues during heart attack, stroke, and transplantation. To reach inflammatory sites, neutrophils must first recognize adhesion molecules of endothelial cells lining the circulatory system. Subsequently, neutrophils efficiently cross tissue planes, basement membranes, and interstitial tissues to reach inflammatory foci. Spontaneous neutrophil locomotion involves a series of coordinated cellular processes including oscillatory changes in integrin adhesiveness, signaling, pericellular proteolysis, oxidant production, and actin assembly (1–9). Directed locomotion or chemotaxis additionally involves the ligation of specific chemotaxin receptors, such as the formyl peptide receptors (10). For example, ligation of the formyl peptide receptor leads to the activation of cellular G protein-coupled signaling pathways (11). However, how the activation of G protein pathways promotes chemotaxis and how cells process chemoattractant information during locomotion are uncertain (12, 13).
To begin to dissect the problem of neutrophil chemoattractant signal processing, in contrast to simple transmembrane chemical reactions, we have studied cell polarization, the first cellular step in neutrophil locomotion. During polarization for locomotion, neutrophils generate well defined morphological features, including a lamellipodium at the leading edge and a uropod at the trailing end. To focus on the temporal component of signal processing, we used a stopped-flow microscopy chamber to remove the spatial component or chemoattractant gradient from the analysis. Furthermore, our previous studies of the oscillatory signal transduction system of the neutrophil suggested a time scale for signal processing (3). Our results show that neutrophils exhibit a rudimentary memory system in which they are able to “recall” prior chemoattractant concentrations and directions in establishing a new orientation; thus, cells rapidly will reverse their direction when exposed to a temporally decreasing signal of an appropriate frequency.
MATERIALS AND METHODS
Isolation of Cells.
Peripheral blood was collected in heparinized vacuum tubes from normal individuals. Neutrophils were separated by using Ficoll–Hypaque (Sigma) density gradient centrifugation. Cells were washed twice with Hanks’ balanced salt solution (HBSS; GIBCO/BRL) then resuspended at 105 cells/ml and stored on ice until used.
Chemotactic Factor.
The chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (FMLP) (Sigma) was used. A stock solution of 10−7 M FMLP in sterile HBSS was stored at −20°C. Serial dilutions from 10−8 to 10−10 M were made from the stock solution. Each dilution was drawn into a syringe and then incubated for 20 min at 37°C before experiments. For determination of peptide distribution in the stopped-flow chamber, a fluorescent formyl peptide ligand, fluoresceinated N-formyl-nle-leu-phe-nle-tyr-lys (Fl-FNLPNTL; Molecular Probes), was used.
Cell Chamber.
To observe the temporal effects chemotactic ligand exposure, a stopped-flow chamber was constructed that was similar to that described previously (14). A glass slide 1 × 3 × 1/32" was fastened to two glass slides 1 × 1 3/8 × 1/16" by using epoxy. The two glass slides were separated by two pieces of 15-gauge stainless steel tubing that also were attached to the glass slide by using epoxy, which created a chamber for cell observation. Two additional 1 × 1 × 1/32" glass slides were attached at the bottom corners of the glass slide to provide space for translation of the objective of the microscope. Silicon vacuum grease was applied around the chamber formed by the stainless steel tubing and glass slides. A 22-mm glass coverslip then was placed on top of the vacuum grease to form a water-tight bottom for the chamber. An 80-μl cell suspension of 1 × 103 cells/ml was added through the 15-gauge tubing. The chamber was inverted for microscopic examination.
Solutions were injected into the chamber by using a 1 1/2" section of tygon tubing (Tygon R-3603, VWR Scientific) with an inner diameter of 1/16". This tubing then was connected to a 10-way adapter constructed of 15-gauge stainless steel entry and exit ports, soldered to a brass body. Each port on the adapter was connected by tygon tubing to a 5-cc disposable syringe. The exit port of the chamber was connected to tygon tubing leading to a disposal container.
Image Processing.
Microscopy was performed by using a Zeiss axiovert microscope attached to a Perceptics Biovision (Knoxville, TN) and/or a Hamamatsu (Middlesex, NJ) Argus 10 image processing system. Differential interference contrast images were collected by a charge-coupled device camera (model 72; Dage–MTI; Michigan City, IN) and recorded on a Sony Umatic video cassette recorder.
Arithmetic averages of 156 frames were taken at the beginning and end of experiments and then stored on a hard disk. Images were photographed on 35-mm film by using a Polaroid freeze-frame video recorder. Polarization changes from the beginning and end of each injection series were determined by drawing lines through the photomicrographs of polarized cells, thus dividing them sagittally. Transverse lines then were drawn at the centers of the cells. Angular changes of the lamellipodia between these two time frames were measured. Previous studies have noted that pseudopod extension is primarily from the lamellipodium (15). No distinction was made between angular changes to the right or left sides.
Fluorescence Microscopy of Fluid Exchange.
Quantitative microfluorometry was conducted by using a photomultiplier tube that, in turn, was connected to an oscilloscope. Serial dilutions of Fl-FNLPNTL were made from 1-mM stock solutions. Dilutions to the concentrations listed below were injected into the chamber at various time intervals.
RESULTS
Chamber Performance.
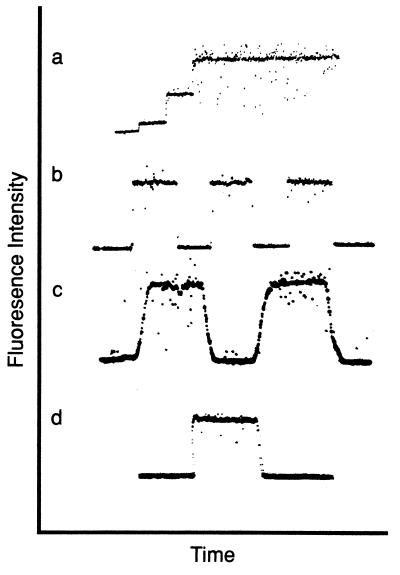
Chamber performance was tested by using various dilutions of Fl-FNLPNTL. After background readings were established on the oscilloscope, Fl-FNLPNTL dilutions from 0 to 3.3 × 10−4 M were injected at 10-s intervals resulting in a stair-step increase in fluorescence intensity (Fig. 1a). The dead space from the 10-way adapter to the end of the chamber was measured to be 200 μl. Fluid velocity during injection was calculated by measuring the distance the fluid moved during a 1-s injection, resulting in a velocity of ≈0.25 m/s over a distance of 7.4 cm. Thus, a change from one concentration to another would be expected to require ≈0.3 s. Quantitatively, this corresponds well with the oscilloscope data of Fig. 1a, where the time separating fluorescence intensity levels was 0.3 s. This sets an upper limit on the time cells were exposed to a spatial gradient because the cell diameter (≈10 μm) is much smaller than the microscopic field (≈200 μm). This result suggests that the solution within the sample chamber is changed rapidly. A shear stress of ≈0.4 dyne/cm2 was calculated for this set-up as described (16), which is within the physiologically relevant region.
Figure 1.
Characterization of the sample chamber by using the fluorescent chemotactic peptide Fl-FNLPNTL. (a) Solutions of increasing peptide concentration were injected into the sample chamber. A stair-step increase in fluorescence intensity is observed by using Fl-FNLPNTL concentrations of 0, 10−4 M, 2.5 × 10−4 M, and 5 × 10−4 M. Rapid increases in signal intensity were observed (n = 10). This trace was recorded while injections were underway to measure the time required for concentration changes. (b) After injection of 10−3 M Fl-FNLPNTL, the fluorescence intensity was measured at different lateral positions spaced 100 μm apart within the sample chamber (n = 13). (The detector was shuttered while moving the microscope stage in traces b and c.) (c) The fluorescence intensity was measured at vertical positions 40 μm apart in the Fl-FNLPNTL (10−3 M)-loaded sample chamber (n = 18). (d) The fluorescence intensity was measured before Fl-FNLPNTL (10−3 M) injection and during chamber washout with HBSS (n = 15). This trace was recorded in real time, which illustrates that the background levels were highly reproducible.
To test for the presence of Fl-FNLPNTL microgradients within the horizontal plane of the chamber, fluorescence intensity readings were made at 100-μm intervals along the long axis of the cell chamber. Identical intensity levels were recorded throughout the axis of the chamber (Fig. 1b). Similar experiments also were performed to test the vertical plane, as illustrated by Fig. 1c. The level of focus was adjusted from the plane of the coverslip to a vertical focus 40 μm into the sample. The square wave appearance of the intensity indicates a stable concentration of Fl-FNLPNTL vertically within the chamber. Similarly, no changes in fluorescence intensity could be detected from side-to-side in the chamber (perpendicular to the long and vertical axes) (data not shown). Next, we tested adsorption of Fl-FNLPNTL to the glass substrate after injection of Fl-FNLPNTL because variations in desorption could cause spatial gradients. After acquiring a baseline on the oscilloscope, an injection of Fl-FNLPNTL followed by an injection of HBSS was performed (Fig. 1d). Similar background readings were recorded before and after injection of Fl-FNLPNTL, corresponding with the intensity levels indicated on the oscilloscope. In all cases, no significant changes in fluorescence intensity were observed, indicating a lack of adsorption under our experimental conditions. These results suggest that no measurable concentration gradients due to flow or absorption were present in the chamber.
Polarization Studies.
The first observable step in neutrophil locomotion is the formation of a lamellipodium and uropod. The polarity of a neutrophil, in turn, determines its future direction of movement (see, e.g., ref. 15). This morphological definition of polarization was used to quantitate the ability of the neutrophil to perceive its environment. The stopped-flow chamber allows the environment of a cell to be changed rapidly without imposing spatial gradients of FMLP. Thus, if a neutrophil is sensing its environment through a spatial mechanism, one would expect only random changes in its direction of polarization. However, a temporal sensing mechanism would cause, during exposure to a decreasing temporal but spatially uniform FMLP concentration, a reverse in directional polarity. Thus, we hypothesized that neutrophils in a temporally decreasing FMLP concentration would perceive that they were migrating in the wrong direction and would reverse their polarity, indicating a proclivity to move in the opposite direction.
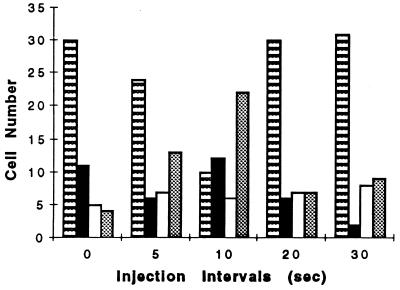
To test this hypothesis, one must know the cellular signal acquisition rate. Presumably, the temporally varying input ligand concentration must be near the cell sampling (signal transduction processing) rate for a cell to detect a temporally decreasing signal and reverse direction. Recent studies from this laboratory have shown that the signal transduction system of migrating neutrophils oscillates with a 10-s period in the presence of FMLP (3, 4). Furthermore, multiple properties of migrating neutrophils oscillate with this period (see, e.g., refs. 1, 2, and 5–9). Therefore, we began by testing multiple injections with a period of 10 s (Fig. 2). The stopped-flow chamber was placed on a 37°C microscopic stage and allowed to warm for 10 min. The injection apparatus containing solutions at 37°C then was attached. By using a ×40 objective, a field of spontaneously polarized cells was selected. Nine concentrations of FMLP from 1 × 10−8 to 1 × 10−10 M were injected at various time intervals (thus yielding total times from 45 to 270 s). Higher concentrations of FMLP (e.g., 10−6 M) were avoided because this concentration triggered extensive degranulation, adherence, and reduced cell motility. The concentration range chosen for these experiments promotes optimal chemokinetic locomotion and cell polarization (see, e.g., ref. 17). Fig. 2 shows the orientations of the cells during decreasing FMLP concentrations. Cells frequently reversed their direction of polarization by 180° with 10-s injections but failed to do so at 20- and 30-s intervals. The Kruskal–Wallis test (18) revealed significant differences (P < 0.001) among the injection period samples. By using nonparametric multiple comparisons, we conclude that the 5- and 10-s injection intervals influence neutrophil turning behavior (P < 0.005). Raw data for the 5- and 10-s injection intervals (without the normalization of Fig. 2) also differed from one another (P < 0.001) as suggested by the Mann–Whitney test. Therefore, injections at a 10-s period were used in further studies.
Figure 2.
Injection frequency-dependence of orientational changes for neutrophils exposed to temporally decreasing FMLP concentrations. The time between sequential injections of FMLP is shown at the abscissa whereas the number of cells is given at the ordinate. The angles of cell reversals are shown for 0 ± 15° (no change or reversal in polarization direction) (striped bars), 45 ± 15° (solid bars), 90 ± 15° (open bars), and 180 ± 15° (dotted bars). A total of 50 cells was measured for each time point. Data for the 0 time interval are for chemokinesis at 10−8 M FMLP. Experiments were performed on 3–19 separate days. Significant differences were found among these populations (see text). These results show that the 10-s injection period was effective in reversing cell orientation by 180°.
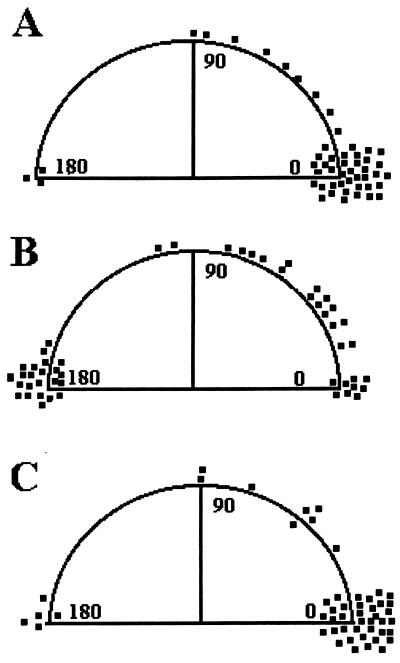
Having established a temporal signal input rate, we next sought to more carefully establish the nature of cell polarization reversal cues. When neutrophils were exposed to 10−8 M FMLP, only approximately one-half of the cells were polarized morphologically; the remainder was adherent (≈45%) or dead (≈5%). A series of FMLP concentrations from 1 × 10−8 to 1 × 10−10 was injected at 10-s intervals followed by an injection of HBSS. The total time for each experiment was ≈2 min. Images of cells were analyzed as described above. Fig. 3 shows a comparison of various pulses. Control experiments were performed by using a series of HBSS pulses (Fig. 3a). We also examined the effects of decreasing and increasing FMLP concentration pulses. P values of >0.001 were obtained in comparing temporally decreasing FMLP concentration pulses to HBSS pulses or in comparing decreasing FMLP concentration pulses to increasing FMLP concentration pulses. The inability of HBSS injections to alter cell polarization shows that the fluid flow associated with injections cannot account for changes in cell orientation. Approximately 44% of the polarized neutrophils reversed direction by 180° ± 15° when exposed to decreasing FMLP concentrations. Representative micrographs of cells undergoing orientation reversals are shown in Fig. 4. As Fig. 3B shows, ≈20% of the neutrophils exposed to a temporally decreasing signal did not change direction (±15° or less). This result could be caused by to a nonresponsive subpopulation of cells. Alternatively, this population may require longer periods of temporally decreasing signals, or the intracellular signaling oscillations (3) could be in a refractile period. Nonetheless, these results do indicate that: (i) many neutrophils respond to a temporally decreasing FMLP signal by changing their orientation, which suggests, in turn, that these cells have a means of remembering environmental FMLP levels and (ii) reorientation is not random but is in general 180° from the original direction of polarization, suggesting directional memory.
Figure 3.
Cell orientational changes during exposure to various solutions in the microscope stopped-flow chamber. (A) Neutrophil orientation changes after pulsed exposure to HBSS in the absence of FMLP. No significant changes are observed. (B) When neutrophils are exposed to a temporally decreasing FMLP signal, most cells reorient at 180° relative to the initial direction of polarization. (C) When neutrophils are exposed to a temporally increasing series of injections, reorientation is not observed. Experiments were replicated on 4–14 separate days.
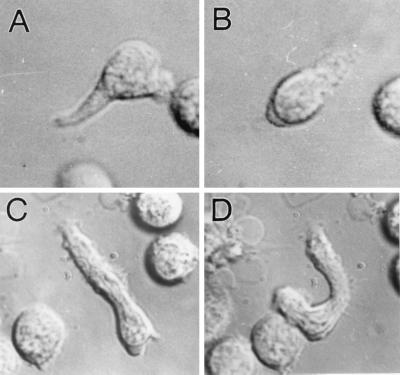
Figure 4.
Examples of cell orientation reversals during exposure due to temporally decreasing FMLP concentrations in a microscope stopped-flow chamber. Cells are shown before (column 1) and after (column 2) exposure to temporally decreasing concentrations of FMLP (10−8 to 10−10 M). Neutrophil orientation generally flips 180° relative to the initial direction of polarization after exposure to a temporally decreasing FMLP signal (i.e., cells detect that they are polarized in a wrong direction). (A and B) An example of a neutrophil reorientating after exposure to a temporally decreasing FMLP signal. In these experiments, cells reversed direction by disassembling their lamellipodia and uropods. (C and D) Occasionally, cells reverse direction by making a “U-turn.” In contrast to these orientational reversals, when neutrophils are exposed to a temporally increasing series of FMLP concentrations or HBSS pulses, reorientation is not observed. Nearby structures provide spatial confirmation of directional reversals. Quantitative data are shown in Fig. 3. (Magnification, ×1,333)
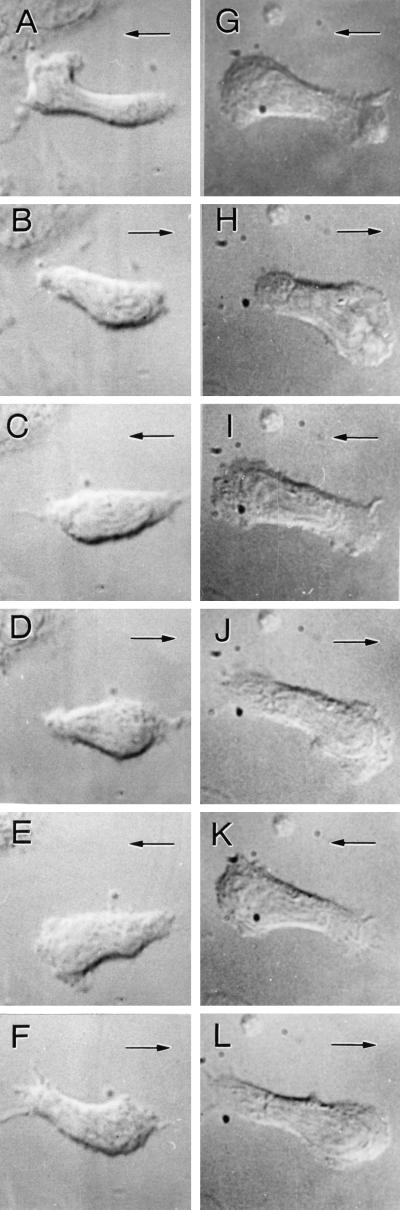
We next examined the reversibility of cell orientational changes by using a sawtooth concentration input profile. Experiments were performed by using decreasing concentrations of FMLP (1 × 10−8 to 1 × 10−10 M and then 0) followed by additional cycles through this same concentration range. Cells were exposed to a decreasing temporal gradient until they exhibited a clear polarization change. The exposure time varied from 40 to 100 s, depending on the rapidity of cell morphological changes. After polarization, reversal was complete (≈1 min), and the cell was subjected to another series of decreasing FMLP concentrations beginning at 1 × 10−8 M, as described above. The second series of injections produced another reversal of polarity. By using this FMLP concentration profile, we could force cells to repetitively flip their polarization (Fig. 5). Of interest, the majority (82%) of cellular polarization changes did not involve a complete “U” turn (18%) (Fig. 4 C and D) but a reversal of polarity within the cell (Figs. 4 and 5). That is, the cell disassembled the lamellipodium and uropod and then reassembled them on the opposite sides. Although a fraction of the cells did not oscillate in this manner, the repeated reversal of a cell to the exact opposite morphological polarity further suggests that a cell coordinates external signals with shape polarity.
Figure 5.
Multiple polarization changes during injection of decreasing FMLP concentrations from 10−8 to 10−10 M at 10-s intervals. Two representative cells from independent experiments are shown in A–F and G–L. The cells flip their morphological polarization five times (each flip corresponds to one “tooth” in the sawtooth injection profile). The directions of cell polarizations (the thick or lamellipodial end of the cell) is noted by the direction of arrows. Note that surrounding materials remain in constant positions, but the cell orientation changes. (Magnification, ×1,266)
DISCUSSION
Although considerable progress has been made in identifying the molecular components of the locomotory signaling apparatus of the neutrophil (10, 11), much uncertainty remains about how these environmental cues are processed to yield coherent cell locomotion. For example, the spatial model proposes that eukaryotic cells simultaneously measure ligand concentrations at the lamellipodium and uropod and then calculate the difference to decide whether to maintain or change their direction of locomotion (12). Temporal models suggest that cells compare chemoattractant levels at different times to yield an output locomotory behavior (13). However, these models are not mutually exclusive; both may contribute to ligand perception (19). In contrast, the temporal aspects of prokaryotic cell movement have been largely sorted out (see, e.g., ref. 20). The present study addresses the more manageable problem of neutrophil morphological polarity, not chemotaxis, by using a stopped-flow chamber to isolate the spatial variable from the temporal variable. We have tested the hypothesis that temporally decreasing FMLP levels trigger polarization reversals in migrating human neutrophils. Our results suggest that a neutrophil exhibits short term memory of both its environmental ligand concentration and direction.
Previous workers have speculated on the presence of an internal clock supporting cell migration (21, 22). We recently proposed that metabolic clocks provide internal control mechanisms for regulating the action and relative phase angles of cellular integrin function, signaling, actin assembly, pericellular proteolysis, and oxidant release (3, 4). This metabolic clock oscillates with a period of 10 or 20 s during cell migration depending on receptor ligation properties. These findings are consistent with neutrophil structure and function oscillations reported by others (1, 2, 5–9). Furthermore, a 10-s signal processing time has been suggested for cell responses to FMLP (23). Because the metabolic clock of the neutrophil oscillates with a 10-s period in the presence of FMLP (3), we speculated that this period also may be the FMLP sampling rate. However, if we inject temporally increasing FMLP concentrations, cells maintain their direction of locomotion (Fig. 3C); thus, we could not be sure that they were responding in a temporal fashion to FMLP. We discovered that application of a temporally decreasing but spatially homogeneous signal caused neutrophils to reverse their direction of migration (Fig. 4). Moreover, when we tested various time intervals between injections, we found that a 10-s period was optimal in stimulating polarization reversal. We recently conjectured that transmembrane signal processing may involve concepts similar to electric circuit theory (3). Thus, the response system of the cell behaves like a filter inasmuch as ligand input frequency determines the physiological output. Furthermore, oscillating receptor/signaling properties offer the advantages of being resistant to noise and maximal gain (24, 25).
Our experimental system revealed several aspects of neutrophil behavior that we believe represent a primitive form of cellular memory. First, cells responded to a temporally decreasing FMLP concentration by reversing direction. This cannot be explained by the presence of spatial gradients of FMLP because such gradients could not be detected over both short and long distances within the observation chamber. Furthermore, it is quite unlikely that some undetected spatial gradient would oscillate by 180° during the repetitive experiments illustrated in Fig. 5. The ability of cells to reverse direction in response to a temporally decreasing signal suggests that cells compare current environmental levels of FMLP to concentrations that they have been exposed to in the recent past. In a physiological setting, this comparison may correspond to the ability of a cell to rapidly change direction to accumulate at inflammatory foci. The ability of a cell to sense incorrect directional cues in cell trafficking is likely to be as important as sensing that they are correct. Second, neutrophils do not randomly select a new direction. Approximately 80% of the motile cells change direction in response to a temporally decreasing chemoattractant signal, and 44% of the total number of motile cells chose 180° ± 15° as their turn angle. In contrast, bacteria randomly select new directions of movement (19). Neutrophil heterogeneity (26) or declining functional responses with age caused by their short lifetimes (≈3 days) may account for the lack of response by 20% of the cells. Thus, in addition to their receptor ligation history, there is also directional memory built into the locomotory apparatus of the neutrophil. One speculative possibility is that one or more proteins at the lamellipodium and uropod do not change places during direction reversals, thus marking sites ≈180° apart. Nonetheless, as pointed out by Zigmond et al. (15), neutrophils exhibit directional responses not shared with bacteria.
Neutrophils reverse direction by at least two means: Either the lamellipodium remains intact and cell migration follows a semicircular path (“U” turn), or the lamellipodium dissolves and reforms at another site. For most cells in this study, the lamellipodium and uropod were disassembled in temporally decreasing FMLP concentrations. These structures then were reassembled at the opposite sides of the cell, thus forming a 180° change in direction. Ramsey (27) previously has noted this type of behavior. However, Zigmond et al. (15) did not observe lamellipodial assembly at the site of a prior uropod. The present study and that of Ramsey (27) used buffers in the absence of a gel matrix whereas Zigmond et al. (15) used a gel matrix. Indeed, for spontaneous migration in gel matrices, our laboratory has not observed lamellopodium–uropod inversion (4). Thus, these differences may reflect the experimental systems.
Neutrophils constitute an extraordinary model system for understanding elementary cell functions. Because the DNA is condensed (giving rise to the term “polymorphonuclear leukocyte”), nuclear signaling is largely unimportant. Neutrophils are a very active cell type; they migrate rapidly, vigorously phagocytose targets, and produce large quantities of reactive oxygen metabolites to destroy targets. Similarly, enucleated neutrophil cytoplasts display all of these functions, particularly migration and chemotaxis (28, 29). Thus, physico-chemical properties of the cytosol should be sufficient to explain the behavioral properties of the neutrophil.
The present study contributes to our understanding of how environmental cues are processed by living cells to stimulate directional changes. Although the lack of spatial gradients in our experiments does not mimic in vivo conditions, the point is to understand how signals are handled by cells to make physiological decisions. By better understanding the physical and chemical nature of neutrophil signal processing, it may become possible to design new ways to block the locomotory/activation/signaling apparatus of the neutrophil to mitigate the harmful effects of neutrophil tissue invasion.
Acknowledgments
This work has been supported by National Institutes of Health Grant 1R01-AI27409 to H.R.P.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: HBSS, Hanks’ balanced salt solution; FMLP, N-formyl-methionyl-leucyl-phenylalanine; Fl-FNLPNTL, N-formyl-nle-leu-phe-nle-tyr-lys.
References
- 1.Ehrengruber M U, Coats T D, Deranleau D A. FEBS Lett. 1995;359:229–232. doi: 10.1016/0014-5793(95)00048-e. [DOI] [PubMed] [Google Scholar]
- 2.Hartman R S, Lau K, Chou W, Coats T D. Biophys J. 1994;67:2535–2545. doi: 10.1016/S0006-3495(94)80743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kindzelskii A L, Eszes M M, Todd R F, III, Petty H R. Biophys J. 1997;73:1777–1784. doi: 10.1016/S0006-3495(97)78208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kindzelskii A L, Zhou M-J, Haugland R P, Boxer L A, Petty H R. Biophys J. 1998;74:90–97. doi: 10.1016/S0006-3495(98)77770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruskal B A, Maxfield F R. J Cell Biol. 1987;105:2685–2693. doi: 10.1083/jcb.105.6.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omann G M, Porasik M M, Sklar L A. J Biol Chem. 1989;264:16355–16358. [PubMed] [Google Scholar]
- 7.Omann G M, Rengan R, Hoffman J F, Linderman J J. J Immunol. 1995;155:5375–5381. [PubMed] [Google Scholar]
- 8.Wymann M P, Kernen P, Deranleau D A, Baggiolini M. J Biol Chem. 1989;264:15829–15834. [PubMed] [Google Scholar]
- 9.Wymann M P, Kernen P, Bengtsson T, Andersson T, Baggiolini M, Deranleau D. J Biol Chem. 1989;265:619–622. [PubMed] [Google Scholar]
- 10.Thomas K M, Pyun H Y, Navarro J. J Biol Chem. 1990;265:20061–20064. [PubMed] [Google Scholar]
- 11.Omann G M, Allen R A, Bokoch G M, Painter R G, Traynor A E, Sklar L A. Physiol Rev. 1987;67:285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- 12.Lauffenburger D, Farrell B, Tranquillo R, Kistler A, Zigmond S. J Cell Sci. 1987;88:415–416. doi: 10.1242/jcs.88.4.415. [DOI] [PubMed] [Google Scholar]
- 13.Vicker M G. J Cell Sci. 1989;92:1–4. doi: 10.1242/jcs.92.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Segall J E. J Muscle Res Cell Motil. 1988;9:481–490. doi: 10.1007/BF01738753. [DOI] [PubMed] [Google Scholar]
- 15.Zigmond S H, Levitsky H I, Kreel B J. J Cell Biol. 1981;89:585–592. doi: 10.1083/jcb.89.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence M B, Springer T A. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 17.Maher J, Martell J V, Brantley B A, Cox E B, Niedel J E, Rosse W F. Blood. 1984;64:221–228. [PubMed] [Google Scholar]
- 18.Zar J H. Biostatistical Analysis. 2nd Ed. Englewood Cliffs, NJ: Prentice–Hall; 1984. [Google Scholar]
- 19.Lackie J M. Cell Movement and Cell Behavior. London: Allen & Unwin; 1986. [Google Scholar]
- 20.Koshland D E. Annu Rev Biochem. 1981;50:765–782. doi: 10.1146/annurev.bi.50.070181.004001. [DOI] [PubMed] [Google Scholar]
- 21.Vicker M G. J Cell Sci. 1994;107:659–667. doi: 10.1242/jcs.107.2.659. [DOI] [PubMed] [Google Scholar]
- 22.Boisfleury-Chevance A, Rapp B, Gruler H. Blood Cells. 1989;15:315–333. [PubMed] [Google Scholar]
- 23.Gerisch G, Keller H H. J Cell Sci. 1981;52:1–10. doi: 10.1242/jcs.52.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Rapp P E, Mees A I, Sparrow C T. J Theor Biol. 1981;90:531–544. doi: 10.1016/0022-5193(81)90304-0. [DOI] [PubMed] [Google Scholar]
- 25.Li Y-X, Goldbeter A. Biophys J. 1992;61:161–171. doi: 10.1016/S0006-3495(92)81824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klempner M S, Gallin J I. Blood. 1978;51:659–670. [PubMed] [Google Scholar]
- 27.Ramsey W S. Exp Cell Res. 1972;70:129–139. doi: 10.1016/0014-4827(72)90190-5. [DOI] [PubMed] [Google Scholar]
- 28.Roos D, Voetman A A, Meerhof L J. J Cell Biol. 1983;97:368–377. doi: 10.1083/jcb.97.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyett D E, Malawista S E, van Blarixom G, Melnick D A, Malech H L. J Immunol. 1985;135:2090–2094. [PubMed] [Google Scholar]