Abstract
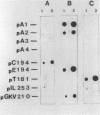
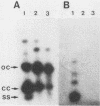
Lactobacillus plantarum A112 has four different plasmids. Plus-origin-specific probes were used to determine that the smallest, cryptic plasmid, pA1 (2,820 bp), showed homology to the pE194 plasmid family. This subclass of plasmids uses the rolling-circle mode of replication. Subsequent analysis of plasmid pA1 demonstrated that it generates single-stranded DNA intermediates, and sequence analysis revealed that it contains three putative open reading frames (ORFs): ORF1, ORF2, and ORF3, which could encode proteins designated RepA (47 amino acids [aa]) and RepB (196 aa) and a protein of 103 aa, respectively. Two of these proteins, RepA (5.6 kDa) and RepB (26 kDa), were identified in in vitro transcription translation assays. The RepA protein contains a characteristic alpha-helix-turn-alpha-helix motif typical of DNA-binding proteins that act as DNA-binding repressors. The RepB protein shows a significant similarity with replication initiation proteins of the pE194 family of plasmids that use the rolling-circle mode of replication. Plasmid pA1 is able to replicate in Escherichia coli and Lactobacillus lactis subsp. lactis as well as in other L. plantarum strains.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates E. E., Gilbert H. J. Characterization of a cryptic plasmid from Lactobacillus plantarum. Gene. 1989 Dec 21;85(1):253–258. doi: 10.1016/0378-1119(89)90491-5. [DOI] [PubMed] [Google Scholar]
- Bergemann A. D., Whitley J. C., Finch L. R. Homology of mycoplasma plasmid pADB201 and staphylococcal plasmid pE194. J Bacteriol. 1989 Jan;171(1):593–595. doi: 10.1128/jb.171.1.593-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boe L., Gros M. F., te Riele H., Ehrlich S. D., Gruss A. Replication origins of single-stranded-DNA plasmid pUB110. J Bacteriol. 1989 Jun;171(6):3366–3372. doi: 10.1128/jb.171.6.3366-3372.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouia A., Bringel F., Frey L., Kammerer B., Belarbi A., Guyonvarch A., Hubert J. C. Structural organization of pLP1, a cryptic plasmid from Lactobacillus plantarum CCM 1904. Plasmid. 1989 Nov;22(3):185–192. doi: 10.1016/0147-619x(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Bron S., Luxen E., Swart P. Instability of recombinant pUB110 plasmids in Bacillus subtilis: plasmid-encoded stability function and effects of DNA inserts. Plasmid. 1988 May;19(3):231–241. doi: 10.1016/0147-619x(88)90041-8. [DOI] [PubMed] [Google Scholar]
- Byeon W. H., Weisblum B. Replication genes of plasmid pE194-cop and repF: transcripts and encoded proteins. J Bacteriol. 1990 Oct;172(10):5892–5900. doi: 10.1128/jb.172.10.5892-5900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Gibson E., Giuffrida A. Evidence for extrachromosomal elements in Lactobacillus. J Bacteriol. 1976 Sep;127(3):1576–1578. doi: 10.1128/jb.127.3.1576-1578.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine K. M., Hogan S. T., Higgins D. G., McConnell D. J. Replication and segregational stability of Bacillus plasmid pBAA1. J Bacteriol. 1989 Feb;171(2):1166–1172. doi: 10.1128/jb.171.2.1166-1172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro M. L., Kornblum J., Novick R. P. A site-specific recombination function in Staphylococcus aureus plasmids. J Bacteriol. 1987 Jun;169(6):2601–2610. doi: 10.1128/jb.169.6.2601-2610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss A. D., Ross H. F., Novick R. P. Functional analysis of a palindromic sequence required for normal replication of several staphylococcal plasmids. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2165–2169. doi: 10.1073/pnas.84.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss A., Ehrlich S. D. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989 Jun;53(2):231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josson K., Soetaert P., Michiels F., Joos H., Mahillon J. Lactobacillus hilgardii plasmid pLAB1000 consists of two functional cassettes commonly found in other gram-positive organisms. J Bacteriol. 1990 Jun;172(6):3089–3099. doi: 10.1128/jb.172.6.3089-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. A., Lopez P., Greenberg B., Espinosa M. Identification and analysis of genes for tetracycline resistance and replication functions in the broad-host-range plasmid pLS1. J Mol Biol. 1986 Dec 20;192(4):753–765. doi: 10.1016/0022-2836(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leenhouts K. J., Tolner B., Bron S., Kok J., Venema G., Seegers J. F. Nucleotide sequence and characterization of the broad-host-range lactococcal plasmid pWVO1. Plasmid. 1991 Jul;26(1):55–66. doi: 10.1016/0147-619x(91)90036-v. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Posno M., Leer R. J., van Luijk N., van Giezen M. J. F., Heuvelmans P. T. H. M., Lokman B. C., Pouwels P. H. Incompatibility of Lactobacillus Vectors with Replicons Derived from Small Cryptic Lactobacillus Plasmids and Segregational Instability of the Introduced Vectors. Appl Environ Microbiol. 1991 Jun;57(6):1822–1828. doi: 10.1128/aem.57.6.1822-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Chopin A. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988 Apr;70(4):559–566. doi: 10.1016/0300-9084(88)90093-4. [DOI] [PubMed] [Google Scholar]
- Sozhamannan S., Dabert P., Moretto V., Ehrlich S. D., Gruss A. Plus-origin mapping of single-stranded DNA plasmid pE194 and nick site homologies with other plasmids. J Bacteriol. 1990 Aug;172(8):4543–4548. doi: 10.1128/jb.172.8.4543-4548.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovo M., Morelli L., Bottazzi V. Drug resistance plasmids in Lactobacillus acidophilus and Lactobacillus reuteri. Appl Environ Microbiol. 1982 Jan;43(1):50–56. doi: 10.1128/aem.43.1.50-56.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F. F., Pearce L. E., Yu P. L. Genetic analysis of a lactococcal plasmid replicon. Mol Gen Genet. 1991 May;227(1):33–39. doi: 10.1007/BF00260703. [DOI] [PubMed] [Google Scholar]
- de la Campa A. G., del Solar G. H., Espinosa M. Initiation of replication of plasmid pLS1. The initiator protein RepB acts on two distant DNA regions. J Mol Biol. 1990 May 20;213(2):247–262. doi: 10.1016/S0022-2836(05)80188-3. [DOI] [PubMed] [Google Scholar]
- del Solar G. H., Puyet A., Espinosa M. Initiation signals for the conversion of single stranded to double stranded DNA forms in the streptococcal plasmid pLS1. Nucleic Acids Res. 1987 Jul 24;15(14):5561–5580. doi: 10.1093/nar/15.14.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Solar G. H., de al Campa A. G., Pérez-Martín J., Choli T., Espinosa M. Purification and characterization of RepA, a protein involved in the copy number control of plasmid pLS1. Nucleic Acids Res. 1989 Apr 11;17(7):2405–2420. doi: 10.1093/nar/17.7.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Solar G., Espinosa M. The copy number of plasmid pLS1 is regulated by two trans-acting plasmid products: the antisense RNA II and the repressor protein, RepA. Mol Microbiol. 1992 Jan;6(1):83–94. doi: 10.1111/j.1365-2958.1992.tb00840.x. [DOI] [PubMed] [Google Scholar]
- te Riele H., Michel B., Ehrlich S. D. Are single-stranded circles intermediates in plasmid DNA replication? EMBO J. 1986 Mar;5(3):631–637. doi: 10.1002/j.1460-2075.1986.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H., Michel B., Ehrlich S. D. Single-stranded plasmid DNA in Bacillus subtilis and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2541–2545. doi: 10.1073/pnas.83.8.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vossen J. M., Kok J., Venema G. Construction of cloning, promoter-screening, and terminator-screening shuttle vectors for Bacillus subtilis and Streptococcus lactis. Appl Environ Microbiol. 1985 Aug;50(2):540–542. doi: 10.1128/aem.50.2.540-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]