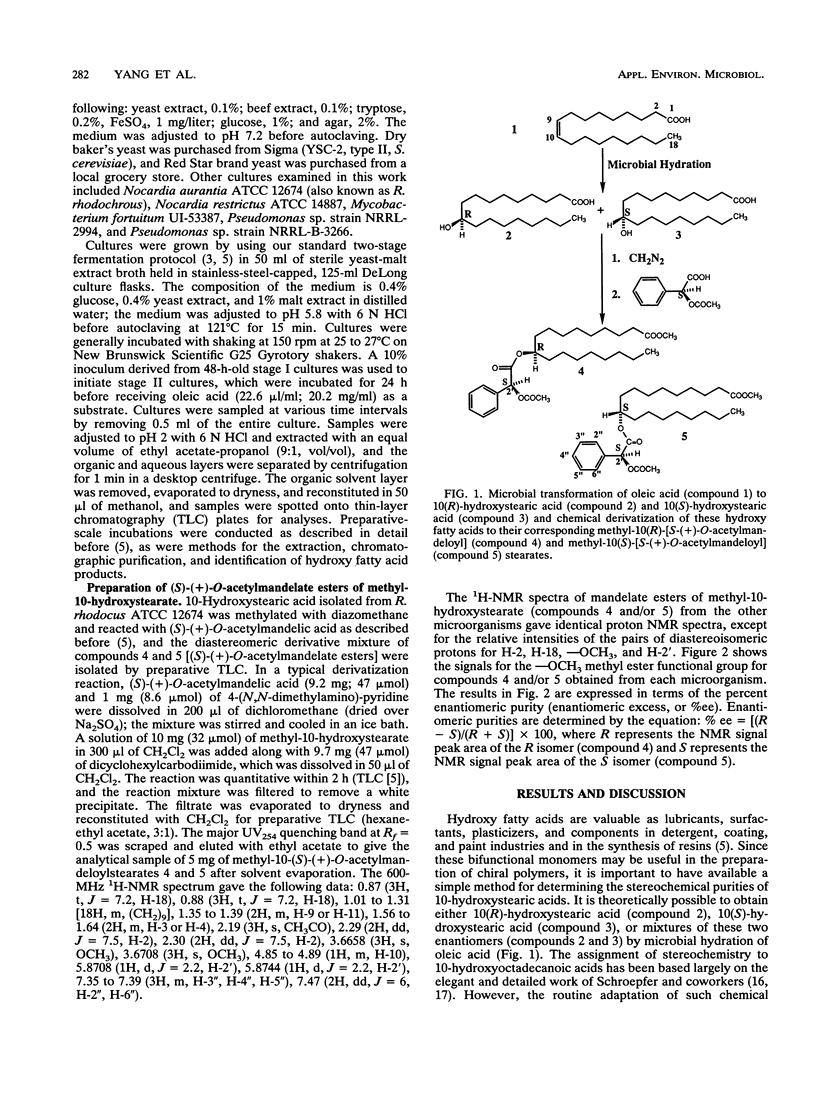
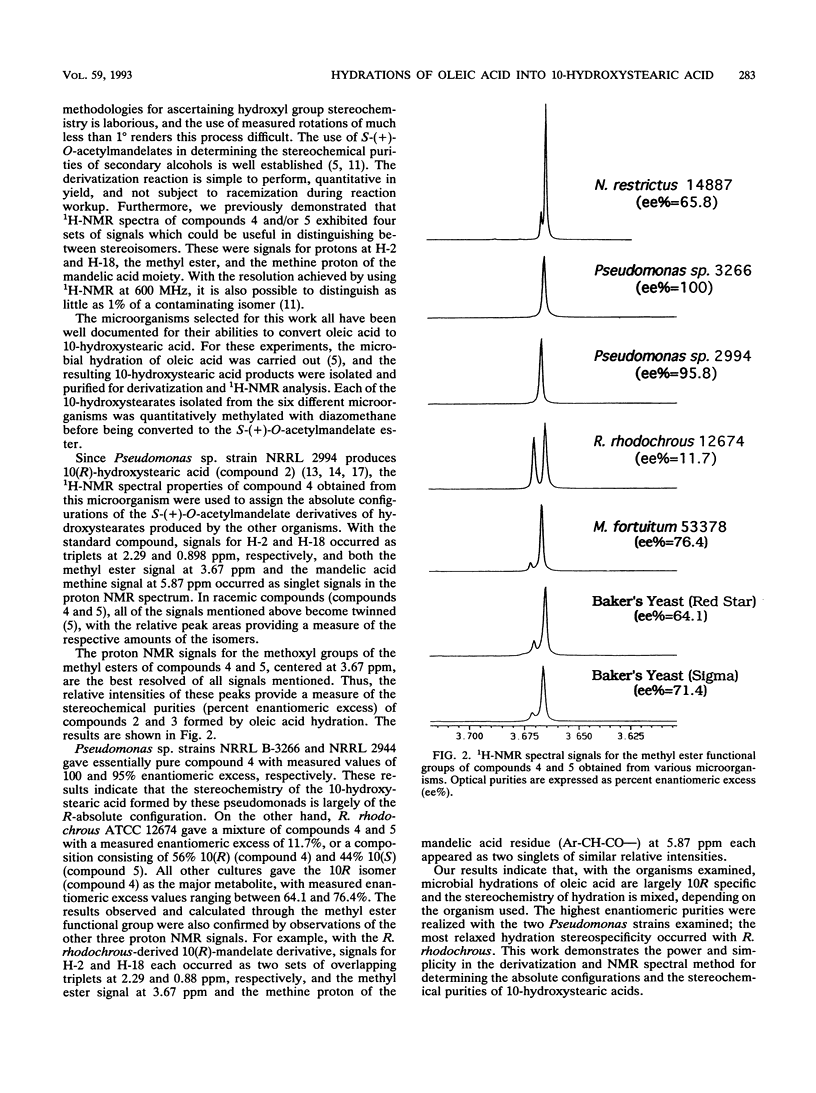
Abstract
We recently described a simple method for ascertaining the stereochemical purities of hydroxy fatty acids (S. H. El-Sharkawy, W. Yang, L. Dostal, and J. P. N. Rosazza, Appl. Environ. Microbiol. 58:2116-2122, 1992) based on the 1H-nuclear magnetic resonance spectral analysis of diastereomeric S-(+)-O-acetylmandelate esters of hydroxystearates. This report describes the stereochemistries of microbial hydrations of oleic acid to 10-hydroxystearic acid by Nocardia aurantia (also known as Rhodococcus rhodochrous) ATCC 12674, Nocardia restrictus ATCC 14887, Mycobacterium fortuitum UI-53387, Pseudomonas species strain NRRL-2994, Pseudomonas species strain NRRL B-3266, and baker's yeast. 10(R)-hydroxystearic acid isolated from Pseudomonas species strain NRRL-2994 was the standard for use in the 1H-nuclear magnetic resonance spectral technique to permit simple assignments of the absolute configurations of 10-hydroxystearic acid produced by different microorganisms. While the R. rhodochrous ATCC 12674-mediated hydration of oleic acid gave mixtures of enantiomers 10(R)-hydroxystearic acid and 10(S)-hydroxystearic acid, Pseudomonas species strain NRRL-B-3266 produced optically pure 10(R)-hydroxystearic acid. The remaining microorganisms stereoselectively hydrated oleic acid to 10(R)-hydroxystearic acid containing between 2 and 18% of the contaminating 10(S)-hydroxystearic acid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betts R. E., Walters D. E., Rosazza J. P. Microbial transformations of antitumor compounds. 1. Conversion of acronycine to 9-hydroxyacronycine by Cunninghamella echinulata. J Med Chem. 1974 Jun;17(6):599–602. doi: 10.1021/jm00252a006. [DOI] [PubMed] [Google Scholar]
- Davis E. N., Wallen L. L., Goodwin J. C., Rohwedder W. K., Rhodes R. A. Microbial hydration of cis-9-alkenoic acids. Lipids. 1969 Sep;4(5):356–362. doi: 10.1007/BF02531006. [DOI] [PubMed] [Google Scholar]
- Mortimer C. E., Niehaus W. G., Jr Enzymatic interconversion of oleic acid, 10-hydroxyoctadecanoic acid, and trans-delta 10-octadecenoic acid. Reaction pathway and stereospecificity. J Biol Chem. 1974 May 10;249(9):2833–2842. [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Schroepfer G. J., Jr The reversible hydration of oleic acid to 10D-hydroxystearic acid. Biochem Biophys Res Commun. 1965 Nov 8;21(3):271–275. doi: 10.1016/0006-291x(65)90282-2. [DOI] [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Torkelson A., Kisic A., Bednarczyk D. J., Schroepfer G. J., Jr Stereospecific hydration of the delta-9 double bond of oleic acid. J Biol Chem. 1970 Aug 10;245(15):3790–3797. [PubMed] [Google Scholar]
- SCHROEPFER G. J., Jr, BLOCH K. THE STEREOSPECIFIC CONVERSION OF STEARIC ACID TO OLEIC ACID. J Biol Chem. 1965 Jan;240:54–63. [PubMed] [Google Scholar]
- SCHROEPFER G. J., Jr ENZYMATIC STEREOSPECIFICITY IN THE CONVERSION OF OLEIC ACID TO 10-HYDROXYSTERARIC ACID. J Am Chem Soc. 1965 Mar 20;87:1411–1412. doi: 10.1021/ja01084a067. [DOI] [PubMed] [Google Scholar]
- Schroepfer G. J., Jr Stereospecific conversion of oleic acid to 10-hydroxystearic acid. J Biol Chem. 1966 Nov 25;241(22):5441–5447. [PubMed] [Google Scholar]
- WALLEN L. L., BENEDICT R. G., JACKSON R. W. The microbiological production of 10-hydroxystearic acid from oleic acid. Arch Biochem Biophys. 1962 Nov;99:249–253. doi: 10.1016/0003-9861(62)90006-1. [DOI] [PubMed] [Google Scholar]
- Wallen L. L., Davis E. N., Wu Y. V., Rohwedder W. K. Stereospecific hydration of unsaturated fatty acids by bacteria. Lipids. 1971 Oct;6(10):745–750. doi: 10.1007/BF02531301. [DOI] [PubMed] [Google Scholar]
- el-Sharkawy S. H., Yang W., Dostal L., Rosazza J. P. Microbial oxidation of oleic acid. Appl Environ Microbiol. 1992 Jul;58(7):2116–2122. doi: 10.1128/aem.58.7.2116-2122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



