Abstract
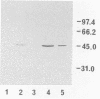
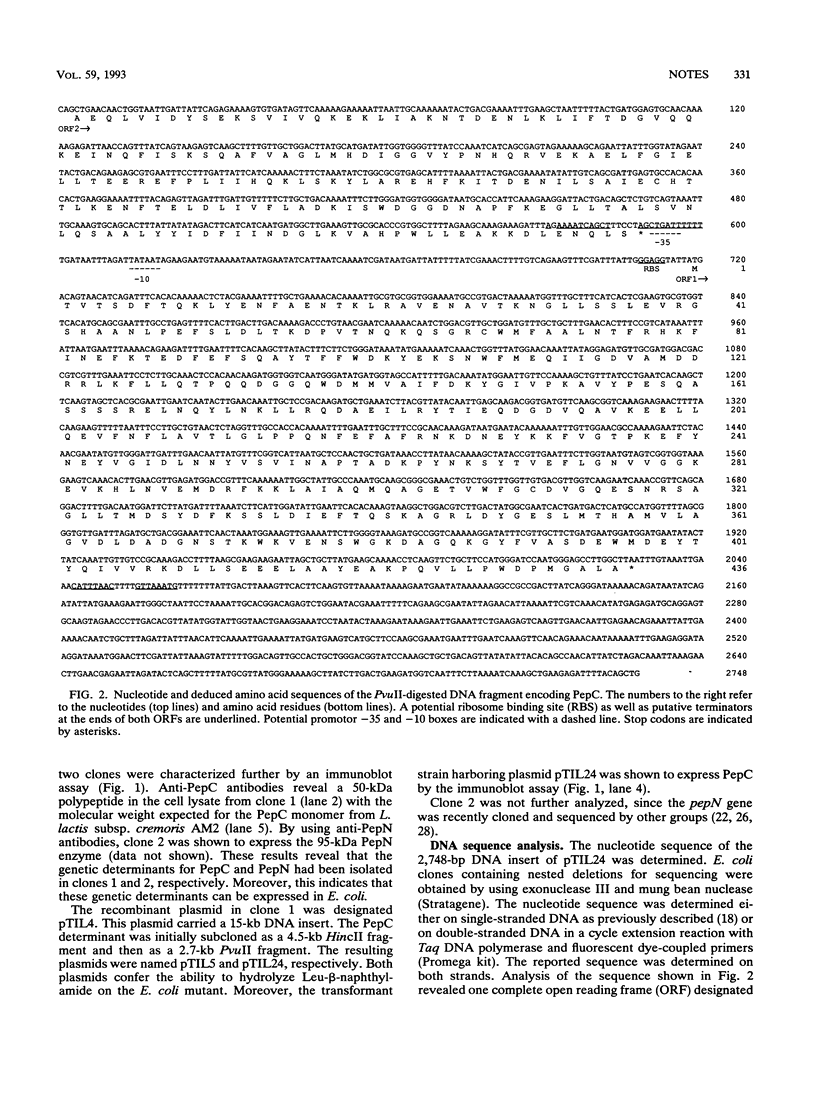
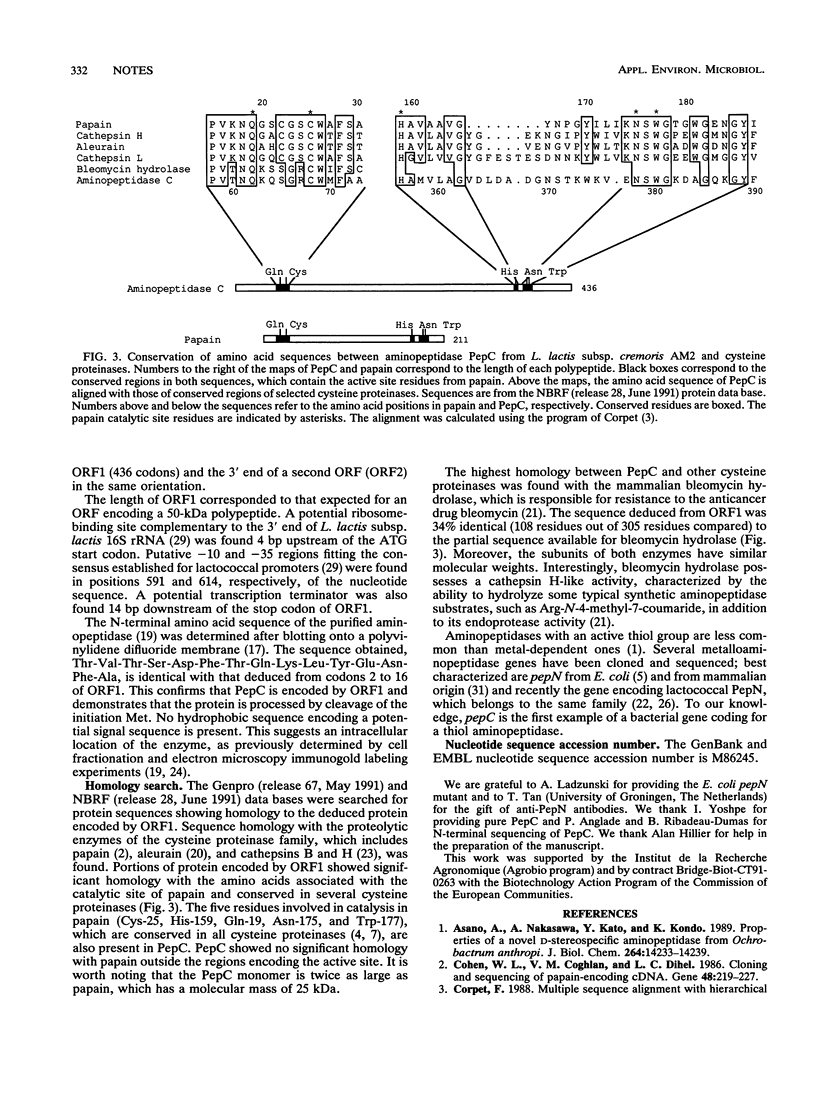
A gene coding for an aminopeptidase (PepC) from Lactococcus lactis subsp. cremoris AM2 was cloned by complementation of an Escherichia coli mutant lacking aminopeptidase activity. The nucleotide sequence was determined. A portion of the predicted amino acid sequence of PepC (436 amino acids) showed strong homology to the active site of cysteine proteases. No signal sequence was found, indicating an intracellular location of the enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano Y., Nakazawa A., Kato Y., Kondo K. Properties of a novel D-stereospecific aminopeptidase from Ochrobactrum anthropi. J Biol Chem. 1989 Aug 25;264(24):14233–14239. [PubMed] [Google Scholar]
- Cohen L. W., Coghlan V. M., Dihel L. C. Cloning and sequencing of papain-encoding cDNA. Gene. 1986;48(2-3):219–227. doi: 10.1016/0378-1119(86)90080-6. [DOI] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour E. Sequence homologies, hydrophobic profiles and secondary structures of cathepsins B, H and L: comparison with papain and actinidin. Biochimie. 1988 Oct;70(10):1335–1342. doi: 10.1016/0300-9084(88)90004-1. [DOI] [PubMed] [Google Scholar]
- Foglino M., Gharbi S., Lazdunski A. Nucleotide sequence of the pepN gene encoding aminopeptidase N of Escherichia coli. Gene. 1986;49(3):303–309. doi: 10.1016/0378-1119(86)90366-5. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Drenth J., Baker E. N. Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985 Mar 20;182(2):317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Kok J. Genetics of the proteolytic system of lactic acid bacteria. FEMS Microbiol Rev. 1990 Sep;7(1-2):15–42. doi: 10.1111/j.1574-6968.1990.tb04877.x. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Poolman B., Driessen A. J. Bioenergetics and solute transport in lactococci. Crit Rev Microbiol. 1989;16(6):419–476. doi: 10.3109/10408418909104474. [DOI] [PubMed] [Google Scholar]
- Laan H., Konings W. N. Autoproteolysis of the Extracellular Serine Proteinase of Lactococcus lactis subsp. cremoris Wg2. Appl Environ Microbiol. 1991 Sep;57(9):2586–2590. doi: 10.1128/aem.57.9.2586-2590.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latil M., Murgier M., Lazdunski A., Lazdunski C. Isolation and genetic mapping of Escherichia coli aminopeptidase mutants. Mol Gen Genet. 1976 Oct 18;148(1):43–47. doi: 10.1007/BF00268544. [DOI] [PubMed] [Google Scholar]
- Mayo B., Kok J., Venema K., Bockelmann W., Teuber M., Reinke H., Venema G. Molecular cloning and sequence analysis of the X-prolyl dipeptidyl aminopeptidase gene from Lactococcus lactis subsp. cremoris. Appl Environ Microbiol. 1991 Jan;57(1):38–44. doi: 10.1128/aem.57.1.38-44.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos M., Jr, Nguyen N. Y., Liu T. Y. Reproducible high yield sequencing of proteins electrophoretically separated and transferred to an inert support. J Biol Chem. 1988 May 5;263(13):6005–6008. [PubMed] [Google Scholar]
- Nardi M., Chopin M. C., Chopin A., Cals M. M., Gripon J. C. Cloning and DNA sequence analysis of an X-prolyl dipeptidyl aminopeptidase gene from Lactococcus lactis subsp. lactis NCDO 763. Appl Environ Microbiol. 1991 Jan;57(1):45–50. doi: 10.1128/aem.57.1.45-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neviani E., Boquien C. Y., Monnet V., Thanh L. P., Gripon J. C. Purification and Characterization of an Aminopeptidase from Lactococcus lactis subsp. cremoris AM2. Appl Environ Microbiol. 1989 Sep;55(9):2308–2314. doi: 10.1128/aem.55.9.2308-2314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C., Dean D., Heck G. R. Aleurain: a barley thiol protease closely related to mammalian cathepsin H. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6512–6516. doi: 10.1073/pnas.82.19.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebti S. M., Mignano J. E., Jani J. P., Srimatkandada S., Lazo J. S. Bleomycin hydrolase: molecular cloning, sequencing, and biochemical studies reveal membership in the cysteine proteinase family. Biochemistry. 1989 Aug 8;28(16):6544–6548. doi: 10.1021/bi00442a003. [DOI] [PubMed] [Google Scholar]
- Strøman P. Sequence of a gene (lap) encoding a 95.3-kDa aminopeptidase from Lactococcus lactis ssp. cremoris Wg2. Gene. 1992 Apr 1;113(1):107–112. doi: 10.1016/0378-1119(92)90676-g. [DOI] [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. S., Chapot-Chartier M. P., Pos K. M., Rousseau M., Boquien C. Y., Gripon J. C., Konings W. N. Localization of peptidases in lactococci. Appl Environ Microbiol. 1992 Jan;58(1):285–290. doi: 10.1128/aem.58.1.285-290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. S., Konings W. N. Purification and Characterization of an Aminopeptidase from Lactococcus lactis subsp. cremoris Wg2. Appl Environ Microbiol. 1990 Feb;56(2):526–532. doi: 10.1128/aem.56.2.526-532.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. S., van Alen-Boerrigter I. J., Poolman B., Siezen R. J., de Vos W. M., Konings W. N. Characterization of the Lactococcus lactis pepN gene encoding an aminopeptidase homologous to mammalian aminopeptidase N. FEBS Lett. 1992 Jul 13;306(1):9–16. doi: 10.1016/0014-5793(92)80827-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P., Boerrigter I. J., Buist G., Haandrikman A. J., Nijhuis M., de Reuver M. B., Siezen R. J., Venema G., de Vos W. M., Kok J. Engineering of the Lactococcus lactis serine proteinase by construction of hybrid enzymes. Protein Eng. 1991 Apr;4(4):479–484. doi: 10.1093/protein/4.4.479. [DOI] [PubMed] [Google Scholar]
- Watt V. M., Yip C. C. Amino acid sequence deduced from a rat kidney cDNA suggests it encodes the Zn-peptidase aminopeptidase N. J Biol Chem. 1989 Apr 5;264(10):5480–5487. [PubMed] [Google Scholar]
- van Alen-Boerrigter I. J., Baankreis R., de Vos W. M. Characterization and overexpression of the Lactococcus lactis pepN gene and localization of its product, aminopeptidase N. Appl Environ Microbiol. 1991 Sep;57(9):2555–2561. doi: 10.1128/aem.57.9.2555-2561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Guchte M., Kok J., Venema G. Gene expression in Lactococcus lactis. FEMS Microbiol Rev. 1992 Feb;8(2):73–92. doi: 10.1111/j.1574-6968.1992.tb04958.x. [DOI] [PubMed] [Google Scholar]