Abstract
Several G protein-coupled receptors are known to direct the tyrosine phosphorylation, and in some cases the activation, of diverse tyrosine kinases. Using a stable cell line approach, we characterize the activation of PYK2, a tyrosine kinase structurally related to focal adhesion kinase, by the G protein-coupled m1 muscarinic acetylcholine receptor. We find that PYK2 tyrosine kinase activity is critical for the m1 receptor-stimulated tyrosine phosphorylation of PYK2. Furthermore, we identify two tyrosine residues that are subject to phosphorylation in response to muscarinic signaling and show that this phosphorylation induces two cytosolic proteins, c-Src and Grb2, to bind to PYK2. This is the first demonstration of the significance played by distinct PYK2 tyrosine residues in G protein-coupled signaling to this kinase. By comparison, though m1 receptors induce the tyrosine phosphorylation of the cytoskeletal protein paxillin, the association of paxillin with PYK2 is unaffected by muscarinic signaling. We also provide evidence that PYK2 specifically phosphorylates the carboxyl-terminal cytosolic portion of the potassium channel Kv1.2 in a manner regulated by the m1 receptor. These results delineate molecular events attending the m1 muscarinic receptor stimulation of this tyrosine kinase and establish PYK2 as an effector of the m1 muscarinic receptor in the regulation of multiple cell functions.
Muscarinic acetylcholine receptors perform crucial functions throughout the central and peripheral nervous systems, controlling many physiological responses to the neurotransmitter acetylcholine (1). The five subtypes of receptors (m1–m5) are members of the family of seven transmembrane receptors, which couple to heterotrimeric guanine nucleotide-binding (G) proteins. The receptor subtypes can be divided into two functional groups. The m1, m3, and m5 subtypes couple to the Gαq/11 class of G proteins to stimulate phospholipase Cβ, which hydrolyzes phosphatidylinositol 4,5-bisphosphate to generate the second messengers diacylglycerol and inositol 1,4,5-trisphosphate (1). These second messengers activate protein kinase C (PKC) and elicit Ca2+ release from intracellular stores, respectively (1). The m2 and m4 receptor subtypes inhibit adenylyl cyclase and activate phospholipases and inward rectifying potassium channels (1, 2).
Several protein tyrosine kinases have been proposed as functional targets of G protein-coupled receptors (GPCRs), including the Src family kinases (3, 4). Indeed the catalytic activities of Src family kinases are elevated by stimulation of Gαq/11-coupled receptors (3, 5). Focal adhesion kinase (FAK) and the related protein tyrosine kinase (PTK) PYK2 have also been proposed as targets of regulation by select GPCRs (3, 4, 6–10). These PTKs are best known for their involvement in the cell-cell and cell-matrix signaling events mediated by cell surface receptors termed integrins (11, 12). Members of the FAK family of tyrosine kinases may participate in T and B cell antigen receptor signaling (12, 13), mitogenesis (14–16), and neuronal signaling events (9, 15). As with the Src family kinases, several neurotransmitters cause an increase in the tyrosine phosphorylation of FAK and PYK2 (4, 6, 8, 10, 17). However, as with the Src family kinases, neither the mechanism by which this signaling occurs nor its relevance for cellular physiology is well understood.
One example of a G protein-coupled neurotransmitter signaling pathway that requires tyrosine kinase activity is the m1 muscarinic acetylcholine (m1) receptor-induced suppression of the delayed rectifier potassium channel Kv1.2 (18, 19). Recently, PYK2 was shown to play a role in the phorbol ester-induced suppression of Kv1.2 (15). It therefore seems plausible that PYK2 may be involved in the tyrosine kinase-dependent suppression of Kv1.2 by m1 receptors. However, it is not known if m1 receptor signaling in any way regulates PYK2. Furthermore, although several GPCRs can induce the tyrosine phosphorylation of PYK2, it has not been shown that any GPCR can regulate the catalytic activity of PYK2. Like the Src family kinases for which tyrosine phosphorylation can be both stimulatory and inhibitory (20), the catalytic activity of FAK family kinases may be independent of tyrosine phosphorylation (21–23).
In studying the activation of a tyrosine kinase by GPCRs two common challenges need to be addressed. On one hand, choosing to study a cell line or a primary tissue preparation that expresses a tyrosine kinase endogenously limits the precision with which one can examine the participation of key structural elements of the kinase in a signaling mechanism. On the other hand, transiently transfecting cells with structurally altered derivatives of the kinase usually results in overexpression of the kinase at levels that surpass the ability of the cell to support its regulation by GPCRs. For example, in cells expressing the m1 muscarinic receptor and overexpressing transiently transfected PYK2 muscarinic stimulation does not affect the phosphorylation state of PYK2 (J.S.F., unpublished observation). Therefore, to investigate whether PYK2 is regulated by the m1 muscarinic acetylcholine receptor, we generated stable cell lines expressing the m1 receptor and epitope-tagged PYK2 variants. These cell lines express PYK2 constructs at stable levels that are consistently regulated by the G protein-coupled m1 muscarinic receptor.
We demonstrate here that m1 receptor stimulation not only leads to the rapid and robust tyrosine phosphorylation of PYK2 but also enhances the catalytic activity of the kinase. This finding contrasts early indications that muscarinic signaling does not stimulate PYK2 (15). Pharmacological analyses indicate that the signal transduction pathway linking m1 receptors to PYK2 may involve both Ca2+ and PKC. We examined the events attending the GPCR regulation of this tyrosine kinase to understand the mechanism by which this signaling occurs. We show that m1 receptor-induced tyrosine phosphorylation of PYK2 depends critically upon the intrinsic catalytic activity of PYK2. We also identify two PYK2 tyrosine residues that are phosphorylated in response to muscarinic signaling and show that these residues support the association between PYK2 and either c-Src or Grb2 in a muscarinic receptor-dependent manner. By comparison, the m1 receptor signaling that induces tyrosine phosphorylation of the cytoskeletal protein paxillin does not affect paxillin’s association with PYK2. Furthermore, we provide evidence that PYK2 specifically phosphorylates the carboxy terminal cytosolic portion Kv1.2 and that this phosphorylation is significantly enhanced by activation of the m1 receptor. These results establish PYK2 as a target of muscarinic signaling with implications for a variety of cell functions.
EXPERIMENTAL PROCEDURES
Stable Expression of PYK2 in Human Embryonic Kidney 293 Cells.
A plasmid [plasmid cytomegalovirus (pCMV)-PYK2-herpes simplex virus (HSV)] encoding rat PYK2 sequence (24) under transcriptional control of the cytomegalovirus early promoter was constructed. Selected point mutations (Y402F, K457A, and Y881F) were introduced into this plasmid by Kunkel mutagenesis, identified by the introduction or removal of a restriction site, and confirmed by sequencing. All PYK2 coding sequences terminated with the HSV epitope, QPELAPEDPED. Human embryonic kidney 293 cells expressing the human m1 receptor were cotransfected with a pCMV-PYK2-HSV plasmid and pBCMGHyg by the Ca2+ phosphate method (18). Clonal lines selected for their resistance to 250 μg/ml hygromycin were screened for their expression of the 115-kDa epitope-tagged construct (PYK2-HSV) by Western blot analysis of cell lysates generated as below. Resulting cell lines were subsequently maintained and propagated as described (18). Cells were starved by reducing the serum content in the media to 0.2% 24 hr before use.
Immunoprecipitations and Western Blot Analysis.
Confluent cultures of stable cell lines were treated with vehicle, atropine, carbachol, phorbol 12-myristate 13-acetate (PMA), or A21387 (Sigma) as indicated at 37°C. Cell lysates were prepared and immunoprecipitated essentially as described (5). PYK2-HSV and paxillin were immunoprecipitated by incubation with 1 μg of anti-HSV (Novagen) or 1 μg of anti-paxillin (Transduction Laboratories, Lexington, KY) at 4°C for 1 hr. After subsequent incubation with protein A Sepharose, the immune complex was pelleted. The immunoprecipitations were washed with lysis buffer and either analyzed by using Western blots or subjected to in vitro kinase assays. Western blot analysis was performed essentially as described (18). Primary anti-HSV, anti-paxillin, anti-c-Src, and anti-Grb2 (Santa Cruz Biotechnology) antibodies were prepared in blocking buffer at 100 ng/ml. Horseradish peroxidase-conjugates of goat anti-mouse or goat anti-rabbit served as the secondary antibody at 1 μg/ml. Phosphotyrosine content was assessed with horseradish peroxidase-conjugated anti-phosphotyrosine antibody 4G10 (Upstate Biotechnology, Lake Placid, NY) diluted to 1 μg/ml.
In Vitro Kinase Assays.
Autophosphorylation reactions, tyrosine kinase assays using the exogenous peptide substrate poly(Glu,Tyr)4:1 (Sigma), and quantification of 32P incorporation were analyzed by SDS/PAGE and autoradiography as described (24). To ascertain that equivalent amounts of kinase were present in each of the autophosphorylation and poly(Glu,Tyr)4:1 kinase reactions, the reaction mixtures were determined by Western blot analysis with antibodies against the HSV epitope. For kinase assays using glutathione S-transferase (GST)-fusion proteins as substrates, the reactions were carried out as above except that immune complex beads were agitated for 10 min at 22°C in 40 μl of freshly prepared buffer containing 40 μg of purified GST-fusion protein, 40 μCi (1 Ci = 37 GBq) of [γ-32P]ATP, 20 mM Tris⋅HCl, 1 mM DTT, 1% Triton X-100, 0.004% Sarcosyl, and 0.1 mM MnCl2 at pH 7.4. These gels were fixed and stained with Coomassie blue before autoradiography.
Preparation of GST-Fusion Proteins.
For bacterial expression of GST-fusion proteins, plasmids encoding either the amino- or carboxy-terminal cytoplasmic regions of rat Kv1.2, were constructed with a PCR strategy that fused the cytosolic amino (amino acids 1–163) and carboxy (amino acids 412–499) termini of Kv1.2 (25) to GST by using the vector pGEX-2T (Pharmacia) to form pGEX-2T-Kv1.2-N and pGEX-2T-Kv1.2-C. These plasmids along with the control pGEX-2T were transformed into E. coli strain BL21 and selected for resistance to 50 μg/ml ampicillin. Cultures inoculated with these bacteria were treated for 2 hr with 0.5 mM isopropyl β-d-thiogalactoside and the induced fusion proteins were solubilized as described (2). Solubilized fusion proteins were applied to a glutathione agarose column, washed, and eluted with 1 mM glutathione. Purified fusion proteins diluted to 5 ml with elution buffer were dialyzed twice against elution buffer lacking glutathione and twice against 20 mM Tris⋅HCl (pH 7.4), 1 mM DTT, 1% Triton X-100, and 0.01% Sarcosyl. The dialysates were centrifuged at 15,000 × g for 20 min at 4°C to remove insoluble material. The supernatants were then concentrated to greater than 2 mg/ml using a Centricon-10 centrifugal concentrator (Amicon).
RESULTS
Stimulation of the m1 Muscarinic Receptor Leads to the Tyrosine Phosphorylation of PYK2.
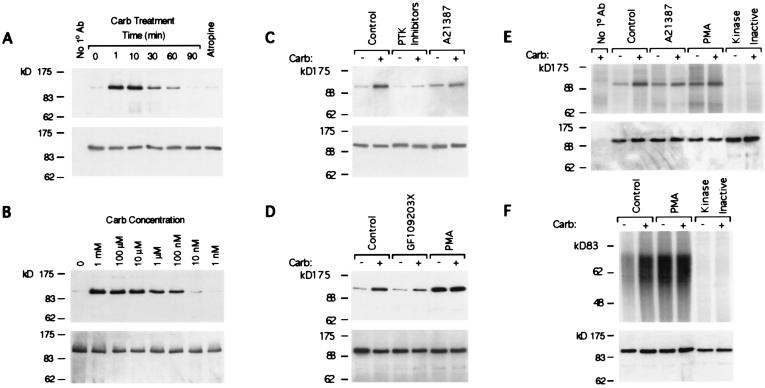
To test whether or not PYK2 was regulated by m1 muscarinic receptors, cells expressing both the human m1 receptor and rat PYK2 bearing an HSV epitope were treated with the muscarinic receptor agonist carbachol. PYK2 protein was immunoprecipitated with anti-HSV antibody from lysates of treated or untreated cells and subjected to Western blot analysis with anti-phosphotyrosine antibody (Fig. 1 A and B Upper). Carbachol caused a striking increase in the level of tyrosine phosphorylated PYK2 (Fig. 1A). Pretreatment with the muscarinic antagonist atropine completely blocked the carbachol-dependent tyrosine phosphorylation of PYK2 (Fig. 1A). The duration of increased PYK2 tyrosine phosphorylation in response to carbachol, which persists for >1 hr, is comparable to the long-lived tyrosine phosphorylation of FAK in response to neuropeptide-activated GPCRs (6). Such a long-lived response contrasts the much shorter-lived GPCR-induced tyrosine phosphorylation of Src family kinases that terminates within minutes (3). Dose response experiments illustrate that the increase in tyrosine phosphorylation of PYK2 in response to carbachol is concentration dependent (Fig. 1B). Furthermore, preincubation with PTK inhibitors significantly diminishes both basal and carbachol-stimulated increases in the phosphotyrosine content of PYK2 relative to untreated controls (Fig. 1C Upper).
Figure 1.
Muscarinic receptor signaling activates PYK2. (A–D) Cells expressing PYK2-HSV were treated as indicated. PYK2 was immunoprecipitated from cell lysates with anti-HSV antibody, and determined by anti-phosphotyrosine Western blot analysis (Upper). (A) Cells were treated at the indicated timepoints with 50 μM carbachol. Controls omitting the primary antibody (Left) or testing atropine pretreatment (1 min, 5 μM; Right) reflect a 10-min carbachol stimulation. (B) Cells were treated with the indicated concentrations of carbachol for 2 min. (C) Cells were either untreated (−) or treated (+) with 50 μM carbachol for 2 min. Pretreatment with PTK inhibitors was for 20 min at these concentrations: genestein, 10 μM; lavendustin A, 1 μM; tyrphostins A23, A25, and A48, 1 μM each. Pretreatment with 5 μM A21387 was for 15 min. (D) Cells were untreated (−) or treated (+) with 50 μM carbachol for 2 min. Pretreatment with 5 μM GF109203X was for 20 min. Pretreatment with 1 μM PMA was for 15 min. (E and F) Autoradiographic analyses of immune complex kinase assays of PYK2 (Upper). Lysates of cell lines expressing either PYK2-HSV or kinase inactive PYK2-HSV prepared before (−) and after (+) 50 μM carbachol stimulation were immunoprecipitated with anti-HSV antibody and the immune complexes used for in vitro kinase assay in the absence (E) or presence (F) of exogenous poly(Glu,Tyr)4:1 polypeptide substrate. Kinase reactions were stopped with 2× SDS/PAGE sample buffer, the mixtures subjected to SDS-PAGE, and the gels dried for autoradiography. In some cases, the cells were pretreated for 15 min with either 5 μM Ca2+ ionophore A21387 (E) or with 1 μM PMA (E and F). A fraction of each immunoprecipitation was reserved for Western blot analysis with anti-HSV antibody to ascertain the equivalency of PYK2 present in immunoprecipitates (A–F Lower).
Pharmacological Analyses of m1 Receptor Signaling to PYK2.
Among the cellular events frequently associated with Gαq/11-coupled GPCRs is the elevation of cytosolic Ca2+ levels and the activation of PKC (1). We therefore examined the role of Ca2+ and PKC in the m1 receptor-dependent stimulation of PYK2 tyrosine phosphorylation (Fig. 1 C and D). Treatment of cells expressing epitope-tagged PYK2 with the Ca2+ ionophore A21387 increased the basal phosphotyrosine content of PYK2 compared with untreated controls (Fig. 1C). However, ionophore treatment failed to evoke a maximal response because subsequent treatment with carbachol further increased PYK2 tyrosine phosphorylation (Fig. 1C). As in previous experiments with other GPCRs (10, 15, 29), pretreating the cells with calcium chelators, such as 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra(acetoxymethyl) ester, significantly diminished the carbachol-induced tyrosine phosphorylation of PYK2 (data not shown). This suggests that while cytosolic Ca2+ likely plays a role, it is only partially responsible for the muscarinic stimulation of PYK2 tyrosine phosphorylation. To investigate the involvement of PKC in the m1 receptor-stimulated elevation of PYK2 phosphotyrosine content, cells were treated with the PKC activator PMA or the PKC inhibitor GF109203X (26) (Fig. 1D). Incubating cells with PMA induced a striking increase in the tyrosine phosphorylation of PYK2 (Fig. 1D) that was not increased by subsequent treatment with carbachol. In contrast, pretreatment of cells with GF109203X significantly attenuated the carbachol response when compared with the response of untreated cells. These results suggest that both PKC and Ca2+ are involved in m1 receptor signaling to PYK2.
Stimulation of m1 Receptors Leads to the Activation of PYK2.
Because stimulation of the m1 receptor leads to the tyrosine phosphorylation of PYK2, we tested whether this correlated with an enhanced catalytic activity of PYK2. We conducted in vitro kinase assays of the enzyme immunoprecipitated from cells expressing PYK2 or cells expressing kinase inactive derivative PYK2-K457A. Immune complexes formed by incubating cell lysates with anti-HSV antibody were assayed for protein kinase activity with [γ-32P]ATP in the absence and presence of an exogenous substrate (Fig. 1E). Immunoprecipitates were generated from cells harvested before and after stimulation with carbachol after incubation with either Ca2+ ionophore A21387, PMA, or vehicle. A protein of ≈115 kDa, the predicted size of the epitope-tagged PYK2, appeared by autoradiography (Fig. 1E). Carbachol stimulation enhances the amount of 32P incorporated by >4-fold. These findings suggest that m1 receptor signaling stimulates PYK2 autophosphorylation. Incubation with Ca2+ ionophore also led to an increase in kinase activity that was modestly augmented by subsequent carbachol incubation (Fig. 1E). Incubation with phorbol ester also increased the kinase activity of PYK2, though to a much greater effect—so much so that further stimulation with carbachol had negligible significance (Fig. 1E). The kinase activity of PYK2 on an exogenous poly(Glu,Tyr)4:1 substrate mirrored PYK2 autophosphorylation responses to carbachol treatment in the presence and absence of phorbol ester (Fig. 1F). In both the autophosphorylation and poly(Glu,Tyr)4:1 assays similar experiments were performed by using cells expressing kinase inactive PYK2-K457A to confirm that no exogenous kinase activity was associated with HSV control immunoprecipitates. Thus, these results show that in response to m1 receptor stimulation PYK2 is not only tyrosine phosphorylated but also activated.
Cell Lines Expressing PYK2 Point Mutant Constructs Reveal Multiple Events Induced by Muscarinic Signaling.
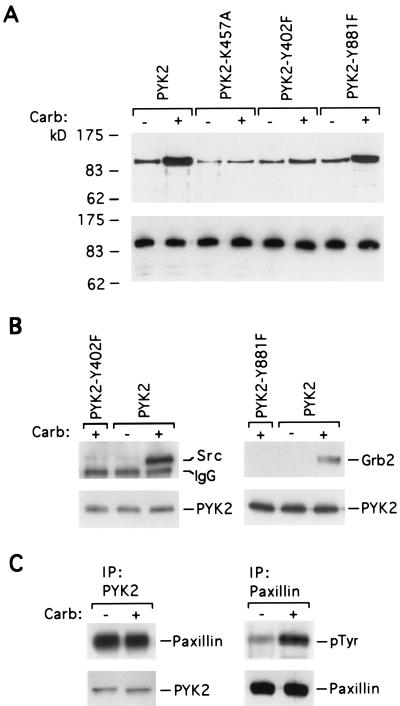
To better understand the molecular events attending activation of PYK2 by the m1 receptor, additional cell lines expressing epitope-tagged PYK2 with selected point mutations were generated. Compared with the tyrosine phosphorylation level of unaltered PYK2, the kinase inactive mutant PYK2-K457A shows basal tyrosine phosphorylation levels that are significantly diminished, suggesting that cellular PYK2 may be subject to autophosphorylation (Fig. 2A). Relative to unaltered PYK2, carbachol-induced tyrosine phosphorylation of kinase inactive PYK2 was also diminished though not abolished. This finding indicates that tyrosine phosphorylation of PYK2 in response to GPCR signaling depends critically upon the intrinsic catalytic activity of PYK2. These results are not complicated by the participation of endogenous PYK2 because human embryonic kidney 293 cells do not express PYK2 that is immunologically detectable (refs. 4 and 27; J.S.F., unpublished observation).
Figure 2.
PYK2 points mutations identify several events regulated by the m1 receptor. (A) Stable cell lines expressing the m1 receptor together with HSV-tagged PYK2, kinase inactive PYK2-K457A, PYK2-Y402F, or PYK2-Y881F were treated for 2 min with vehicle (−) or 50 μM carbachol (+). Lysates from these cell lines were immunoprecipitated with anti-HSV antibody and determined by Western blot analysis with either anti-phosphotyrosine antibody (Upper) or anti-HSV antibody (Lower). (B) Anti-HSV immunoprecipitated lysates of the indicated cell lines prepared before (−) and after (+) carbachol treatment (2 min, 50 μM) were immunoblotted with antibodies against c-Src (Upper Left) or Grb2 (Upper Right). In each case the level of PYK2 present in immunoprecipitates was assessed by Western blot analysis with anti-HSV antibody (Lower). (C) Cells expressing PYK2-HSV were lysed before (−) and after (+) a 2 min 50 μM carbachol stimulation. Anti-HSV immunoprecipitates from the lysates were analyzed by Western blot using anti-paxillin (Upper Left) or anti-HSV (Lower Left) antibodies. Anti-paxillin immunoprecipitates were analyzed by Western blot using anti-phosphotyrosine antibodies (Upper Right) or anti-paxillin (Lower Right).
Because overexpression of PYK2 correlates with elevation of cellular mitogen-activated protein kinase (MAPK) activity and c-Src phosphorylation, PYK2 has been proposed as candidate signaling intermediary linking Src family kinases with GPCR stimulation of MAPK activity (4, 27). This proposal elaborates findings that PYK2 can bind to the Src homology 2 domains of tyrosine kinase c-Src and adaptor protein Grb2, a protein participating in the activation of MAPK (11, 15). However, it is not known how GPCR signaling might regulate the association between PYK2 and either c-Src or Grb2. Therefore, we sought to determine if the m1 receptor could modulate the association between PYK2 and c-Src or Grb2. Previous studies have identified two tyrosine residues that play key roles in FAK and PYK2 signaling (4, 11, 15, 22, 28). Autophosphorylation of FAK occurs at Y397 and this autophosphorylation regulates the association of FAK with Src family kinases (22, 28). By homology to FAK, Y402 is the predicted autophosphorylation site of PYK2 and has been shown to mediate the association of c-Src with PYK2 (4). Similarly, by analogy to studies on FAK, PYK2 Y881 is predicted to be the site of Grb2 association with PYK2 (11, 15). However, it has not been shown that either Y402 or Y881 plays any role in the regulation of PYK2 by GPCRs.
To test the involvement of these tyrosines in the m1 receptor regulation of PYK2 we generated stable cell lines expressing PYK2 variants with Tyr-402 or -881 mutated to phenylalanine. When PYK2 is immunoprecipitated from these cells lines before and after carbachol stimulation, anti-phosphotyrosine Western blot analysis reveals that both mutations diminish basal and carbachol-induced tyrosine phosphorylation of PYK2, though the effect of the Y881F mutation is modest (Fig. 2A). Like the kinase inactivating mutation K457A, neither mutation blocks a carbachol-stimulated increase of PYK2 tyrosine phosphorylation. Mutation of Y402 seems to hinder PYK2 tyrosine phosphorylation more dramatically than mutation of Y881, indicating that phosphorylation of this tyrosine is more dominant.
To further characterize the role of these tyrosine residues in m1 receptor signaling to PYK2, we assessed the ability of PYK2 to bind c-Src and Grb2 in response to carbachol. Muscarinic signaling induces the association of both c-Src and Grb2 with PYK2 (Fig. 2B). Both Y402 and Y881 appear to participate in the GPCR regulation of PYK2 because their mutation ablates the m1 receptor-dependent association of PYK2 with c-Src or Grb2, respectively. By comparison, muscarinic signaling does not affect the association of PYK2 with cytoskeletal protein paxillin (Fig. 3C). Paxillin is known to associate with PYK2 (29, 30) and can serve as a substrate for FAK family kinases (30, 31). Indeed, as with the G protein-coupled angiotensin II receptor (29) the m1 receptor does elicit the tyrosine phosphorylation of paxillin in these cell lines (Fig. 2C). It seems likely that m1 receptor regulation of c-Src and paxillin involves PYK2 because stimulation of this GPCR activates PYK2 and elevates the phosphotyrosine levels of both PYK2-binding proteins paxillin and c-Src (5).
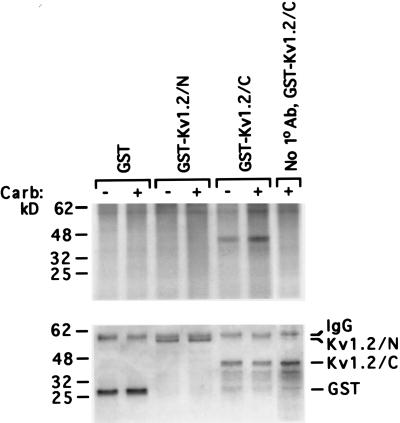
Figure 3.
Muscarinic receptor signaling regulates the ability of PYK2 to phosphorylate the carboxy terminus of Kv1.2. (A) Autoradiograph of immune complex PYK2 kinase assays of PYK2 immunoprecipitated from cells expressing PYK2-HSV. Lysates prepared before (−) and after (+) carbachol stimulation (2 min, 50 μM) were mixed with purified GST, the cytosolic Kv1.2 amino terminus purified as a GST-fusion protein (GST-Kv1.2/N), or the cytosolic Kv1.2 carboxy terminus purified as a GST-fusion protein (GST-Kv1.2/C) in the presence of [γ-32P]ATP and kinase buffer. Control assays with the GST-Kv1.2/C substrate omitted the primary anti-HSV antibody. (B) The same gel visualized by Coomassie blue staining.
PYK2 Isolated from Carbachol-Treated Cells Phosphorylates the Carboxyl-Terminal Cytosolic Region of Kv1.2.
Recent evidence suggests that PYK2 may play a role in suppressing the ionic currents generated by the delayed rectifier potassium channel Kv1.2 because a kinase-inactive derivative of PYK2 blocks the PMA-induced suppression of Kv1.2 activity in Xenopus oocytes (15). Though it is clear that Kv1.2 is suppressed by signaling from GPCRs, it remains unknown whether the ability of PYK2 to phosphorylate Kv1.2 is regulated by any GPCR. We have shown (18) that the activity of Kv1.2 is suppressed by m1 receptors via a mechanism involving tyrosine phosphorylation of the potassium channel in regions predicted to lie on the cytoplasmic side of the channel. Having shown that m1 receptors activate PYK2, we assessed the ability of PYK2 to directly phosphorylate the cytosolic portions of Kv1.2 in a regulated manner (Fig. 3). PYK2 extracted from cells before and after carbachol stimulation was washed extensively and mixed with either GST alone, GST fused to the Kv1.2 amino-terminal cytoplasmic domain (GST-Kv1.2/N), or GST bearing the Kv1.2 carboxyl-terminal cytoplasmic residues (GST-Kv1.2/C). The purified GST, GST-Kv1.2/N, and GST-Kv1.2/C proteins were ≈27, 54, and 43 kDa, respectively. Interestingly, PYK2 isolated from carbachol-treated cells phosphorylated only the Kv1.2 carboxy terminus fusion protein (Fig. 3A). On average, there was nearly a four fold increase in the amount of 32P incorporated into the Kv1.2 carboxyl-terminal fusion protein mixed with carbachol-activated PYK2 over PYK2 from controls cells. Neither GST alone nor the Kv1.2 amino-terminal fusion protein showed any significant 32P incorporation. In each experiment the gel used to assess PYK2 kinase activity was stained with Coomassie blue to confirm that equal amounts of substrate protein were loaded onto the gel for comparison (Fig. 3B).
DISCUSSION
This study establishes for the first time that m1 receptors elicit not only the increased tyrosine phosphorylation but also the catalytic activation of PYK2. Pharmacological experiments suggest that PKC and Ca2+ may play a role in this response. Receptor stimulation of PYK2 enhanced the catalytic activity of the tyrosine kinase in autophosphorylation reactions and in assays using either a generic polypeptide substrate or a GST-fusion protein bearing the carboxyl-terminal cytosolic portion of the potassium channel Kv1.2. The ability of PYK2 to phosphorylate the carboxy-terminal portion of Kv1.2 appears specific: neither a GST-fusion protein with the amino-terminal cytosolic portion of Kv1.2 nor GST alone served as a substrate for PYK2. Previous reports implicated PYK2 in the phosphorylation-dependent regulation of Kv1.2 in phorbol ester-treated Xenopus oocytes (15), and our report extends this work in two important ways. First, we confirm that the ability of PYK2 to phosphorylate the channel is regulated by a natural Kv1.2-modulating receptor. Second, we delineate the region of the ion channel on which PYK2 acts directly.
Moreover, we have determined that the catalytic activity of PYK2 and two key tyrosine residues play important roles in muscarinic receptor signaling to PYK2. The m1 receptor regulates the association of PYK2 with both c-Src and Grb2. However, while muscarinic signaling induces tyrosine phosphorylation of the cytoskeletal protein paxillin, this response does not ablate the association between PYK2 and paxillin. Earlier evidence indicated that c-Src and Grb2 interact with PYK2 at Y402 and Y881, respectively (4, 11); however, these experiments relied on either in vitro studies of GST-fusion proteins or transient overexpression in transfected cells. Our work using stable cell lines demonstrates that interaction of these signaling proteins can occur in cells expressing c-Src, Grb2, and PYK2 at natural levels in a manner responsive to extracellular stimulus. This is more physiologically informative because only under such conditions do we see these interactions regulated by a defined GPCR. In sum, these findings demonstrate that a GPCR can regulate the catalytic activity of PYK2 and in characterizing how this occurs, we provide evidence that m1 receptor activation of PYK2 can impinge on diverse functions within the same cell.
The exact route by which PYK2 is activated by m1 receptors or other GPCRs is unknown. Covalent post-translational modification could account for the ability of PYK2 to display increased kinase activities when isolated by immunoprecipitation. Because GPCRs elicit the tyrosine phosphorylation of both FAK and PYK2, phosphotyrosine modulation of PYK2 catalytic activity receives first consideration (4, 6, 7, 11). Careful work examining the significance of tyrosine phosphorylation in regulating FAK catalytic activity serves as the best available model in considering the issue for PYK2 (22, 23). The evidence suggests that FAK activity may not always correlate with tyrosine phosphorylation (21–23). The issue is complicated by the multiplicity of tyrosine phosphorylation sites present in FAK family kinases (23). Discerning the role of these phosphotyrosine sites is important because the regulation of PYK2 kinase activity may be analogous to the better-studied tyrosine kinase c-Src for which tyrosine phosphorylation at different sites can be either stimulatory or inhibitory (20). We note that the stable cell line approach we have taken is especially valuable in studying the structural components of PYK2 most critical for its GPCR-dependent regulation. The evidence we present indicates that multiple PYK2 tyrosine phosphorylation events occur in response to m1 receptor signaling and that stimulated PYK2 autophosphorylation performs a function integral to this signaling mechanism. Because the same GPCR stimulation that activates PYK2 also induces the association between PYK2 and c-Src, the participation of a Src family kinase in this signaling mechanism also seems likely.
Our demonstration that m1 receptors activate PYK2 has implications for many cellular processes, including the regulation of MAPK, cytoskeleton, and ion channel functions. The results presented here elaborate proposals that PYK2 is a likely participant in the GPCR regulation of MAPK signaling in that m1 receptor stimulation regulates an association between PYK2 and Grb2. This GPCR regulated intermolecular association is proposed to be suitable for subsequent activation of MAPK activity (4). Indeed, it is known that m1 receptor signaling plays an important role in stimulating cellular proliferation, also an outcome of MAPK activation (17). By comparison, paxillin is a cytoskeletal protein that is bound constitutively and phosphorylated by both FAK and PYK2 (30, 31). It seems plausible that the muscarinic activation of PYK2 may lead to paxillin tyrosine phosphorylation and remodel paxillin’s associations with other cytoskeletal proteins (30, 32). Modulation of cytoskeletal events may also involve adhesive interactions mediated by cell surface receptors important in directing cellular proliferation, migration, and differentiation (33). PYK2, like FAK, is involved in signaling processes governed by adhesive receptors called integrins (11, 12, 34). A recognized aspect of FAK family kinase signaling, GPCR regulation of kinases also targeted by integrin signaling establishes a point for the convergence of different signal transduction pathways (33, 35). If integrin and GPCR signals must combine to generate a PYK2 response absent with either stimulus alone, the kinase would provide a site of coincidence detection—analogous to costimulatory signals acting on FAK in platelets (7). The possibility that integrin and muscarinic signaling to PYK2 combine to modulate the cytokinetic processes involved in cellular proliferation therefore merits further study.
The modulation of Kv1.2 through direct phosphorylation by PYK2 further expands the significance of GPCR regulation of this tyrosine kinase. The kinase-dependent regulation of this potassium channel is well established. For example, the activity of the potassium channel Kv1.2 is known to be strongly enhanced by the direct action of serine/threonine specific protein kinase A (36). In contrast, Kv1.2 activity is suppressed by m1 receptors by a mechanism involving tyrosine kinases (18, 19). Both of these kinase activities are known to act upon regions of the amino-terminal cytoplasmic region of Kv1.2. Our demonstration that m1 receptors activate PYK2, and that PYK2 can directly phosphorylate the carboxy terminus of Kv1.2 in a fashion regulated by the m1 receptor imparts a new mechanism for the regulation of this potassium channel. It seems likely that tyrosine kinases acting on both the amino- and carboxy-terminal cytosolic portions of the channel may be involved in regulating its activity. Consistent with this hypothesis are electrophysiological analyses of Kv1.2 currents recorded in human embryonic kidney cells and in Xenopus oocytes confirming that tyrosine residues in both the amino and carboxy-terminal cytosolic regions are critical to the muscarinic regulation of the channel (T.G.C. and A. D. Morielli, unpublished data). Indeed, recent evidence suggests that other potassium channels in the Kv1.2 family physically associate with PTKs in a manner that may be supported by cytoskeletal scaffolding proteins (37, 38). Further investigation will be needed to understand the cellular components mediating the tyrosine kinase-dependent regulation of Kv1.2.
Acknowledgments
We thank Dr. Terakatsu Sasaki for the plasmid encoding rat PYK2. We also thank Dr. Anthony D. Morielli and other members of our lab for helpful discussions during the preparation of this manuscript. This work was supported by a predoctoral training grant from the National Institutes of Health (J.S.F.) and in part by a postdoctoral fellowship from the Ministerio de Educación y Ciencia, Spain (T.G.C.).
ABBREVIATIONS
- FAK
focal adhesion kinase
- GPCR
G protein-coupled receptor
- GST
glutathione S-transferase
- HSV
herpes simplex virus
- MAPK
mitogen-activated protein kinase
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PTK
protein tyrosine kinase
References
- 1.Ashkenazi A, Peralta E G. CRC Handbook of Receptors and Channels. Vol. 1. Boca Raton, FL: CRC; 1994. pp. 1–27. [Google Scholar]
- 2.Kunkel M T, Peralta E G. Cell. 1995;83:443–449. doi: 10.1016/0092-8674(95)90122-1. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y H, Pouyssegur J, Courtneidge S A, Van Obberghen Schilling E. J Biol Chem. 1994;269:27372–27377. [PubMed] [Google Scholar]
- 4.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. Nature (London) 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 5.Tsai W, Morielli A D, Peralta E G. EMBO J. 1997;16:4597–4605. doi: 10.1093/emboj/16.15.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zachary I, Sinnett Smith J, Rozengurt E. J Biol Chem. 1992;267:19031–19034. [PubMed] [Google Scholar]
- 7.Shattil S J, Haimovich B, Cunningham M, Lipfert L, Parsons J T, Ginsberg M H, Brugge J S. J Biol Chem. 1994;269:14738–14745. [PubMed] [Google Scholar]
- 8.Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor L M, White R A, Groopman J E, et al. J Biol Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- 9.Siciliano J C, Toutant M, Derkinderen P, Sasaki T, Girault J-A. J Biol Chem. 1996;271:28942–28946. doi: 10.1074/jbc.271.46.28942. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Li X, Marchetto G S, Dy R, Hunter D, Calvo B, Dawson T L, Wilm M, Anderegg R J, Graves L M, Earp H S. J Biol Chem. 1996;271:29993–29998. doi: 10.1074/jbc.271.47.29993. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Avraham H, Rogers R A, Raja S, Avraham S. Blood. 1996;88:417–428. [PubMed] [Google Scholar]
- 12.Astier A, Avraham H, Manie S N, Groopman J, Canty T, Avraham S, Freedman A S. J Biol Chem. 1997;272:228–232. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- 13.Manie S N, Beck A R, Astier A, Law S F, Canty T, Hirai H, Druker B J, Avraham H, Haghayeghi N, Sattler M, et al. J Biol Chem. 1997;272:4230–4236. doi: 10.1074/jbc.272.7.4230. [DOI] [PubMed] [Google Scholar]
- 14.Guan J L, Shalloway D. Nature (London) 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- 15.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Lin T H, Der C J, Juliano R L. J Biol Chem. 1996;271:18122–18127. doi: 10.1074/jbc.271.30.18122. [DOI] [PubMed] [Google Scholar]
- 17.Gutkind J S, Crespo P, Coso O A, Kanilec G, Xu N. In: Molecular Mechanisms of Muscarinic Acetycholine Receptor Function. Wess J, editor. Austin, TX: Landes; 1995. pp. 103–142. [Google Scholar]
- 18.Huang X Y, Morielli A D, Peralta E G. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- 19.Morielli A D, Huang X-Y, Peralta E G. In: Molecular Mechanisms of Muscarinic Acetycholine Receptor Function. Wess J, editor. Austin, TX: Landes; 1995. pp. 143–164. [Google Scholar]
- 20.Cooper J A, Howell B. Cell. 1993;73:1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- 21.Chan P Y, Kanner S B, Whitney G, Aruffo A. J Biol Chem. 1994;269:20567–20574. [PubMed] [Google Scholar]
- 22.Eide B L, Turck C W, Escobedo J A. Mol Cell Biol. 1995;15:2819–2827. doi: 10.1128/mcb.15.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calalb M B, Polte T R, Hanks S K. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki H, Nagura K, Ishino M, Tobioka H, Kotani K, Sasaki T. J Biol Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- 25.Paulmichl M, Nasmith P, Hellmiss R, Reed K, Boyle W A, Nerbonne J M, Peralta E G, Clapham D E. Proc Natl Acad Sci USA. 1991;88:7892–7895. doi: 10.1073/pnas.88.17.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 27.Della Rocca G J, van Biesen T, Daaka Y, Luttrell D K, Luttrell L M, Lefkowitz R J. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 28.Schaller M D, Hildebrand J D, Shannon J D, Fox J W, Vines R R, Parsons J T. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Earp H S. J Biol Chem. 1997;272:14341–14348. doi: 10.1074/jbc.272.22.14341. [DOI] [PubMed] [Google Scholar]
- 30.Salgia R, Avraham S, Pisick E, Li J L, Raja S, Greenfield E A, Sattler M, Avraham H, Griffin J D. J Biol Chem. 1996;271:31222–31226. doi: 10.1074/jbc.271.49.31222. [DOI] [PubMed] [Google Scholar]
- 31.Bellis S L, Miller J T, Turner C E. J Biol Chem. 1995;270:17437–17441. doi: 10.1074/jbc.270.29.17437. [DOI] [PubMed] [Google Scholar]
- 32.Hildebrand J D, Schaller M D, Parsons J T. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark E A, Brugge J S. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 34.Richardson A, Parsons J T. Bioessays. 1995;17:229–236. doi: 10.1002/bies.950170309. [DOI] [PubMed] [Google Scholar]
- 35.Zachary I, Rozengurt E. Cell. 1992;71:891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]
- 36.Huang X Y, Morielli A D, Peralta E G. Proc Natl Acad Sci USA. 1994;91:624–628. doi: 10.1073/pnas.91.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmes T C, Fadool D A, Ren R, Levitan I B. Science. 1996;274:2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- 38.Hanada T, Lin L, Chandy K G, Oh S S, Christi A H. J Biol Chem. 1997;272:26899–26904. doi: 10.1074/jbc.272.43.26899. [DOI] [PubMed] [Google Scholar]