Abstract
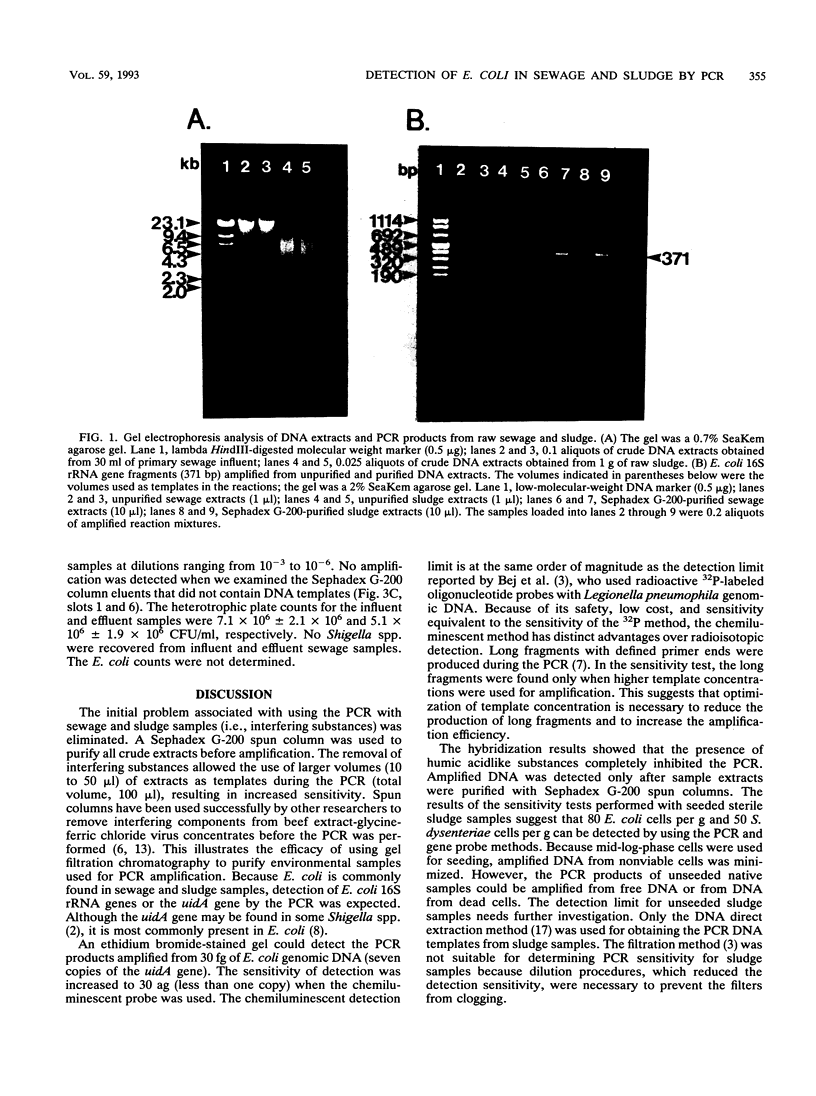
A method in which the polymerase chain reaction (PCR) was used was developed to amplify either a uidA gene fragment or a 16S rRNA gene fragment from Escherichia coli in sewage and sludge. Because of interference caused by humic acidlike substances, crude DNA extracts were purified with a Sephadex G-200 spun column before the PCR was begun. A Southern analysis in which a nonradioactive chemiluminescent method was used was performed to confirm the presence of PCR products. The sensitivity of detection for PCR products when the chemiluminescent method was used was determined to be 30 ag of E. coli genomic DNA template. In seeded sludge, the PCR amplified the target DNA from 80 E. coli cells per g of sludge and 50 Shigella dysenteriae cells per g of sludge. Because only 0.05 aliquot of a sludge extract was used for the PCR, we deduced that the PCR detected target DNA equivalent to the DNA of 2.5 to 4 cells in the extract. The PCR amplified the uidA fragment from diluted sewage influents and effluents containing E. coli cells. Therefore, the PCR performed with a chemiluminescent gene probe can be used to detect the presence of potentially pathogenic microorganisms in sewage and sludge. This technique can be expanded to permit direct detection of pathogenic microorganisms in water samples, thus leading to enhanced public health protection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bej A. K., DiCesare J. L., Haff L., Atlas R. M. Detection of Escherichia coli and Shigella spp. in water by using the polymerase chain reaction and gene probes for uid. Appl Environ Microbiol. 1991 Apr;57(4):1013–1017. doi: 10.1128/aem.57.4.1013-1017.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bej A. K., Mahbubani M. H., Dicesare J. L., Atlas R. M. Polymerase chain reaction-gene probe detection of microorganisms by using filter-concentrated samples. Appl Environ Microbiol. 1991 Dec;57(12):3529–3534. doi: 10.1128/aem.57.12.3529-3534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauns L. A., Hudson M. C., Oliver J. D. Use of the polymerase chain reaction in detection of culturable and nonculturable Vibrio vulnificus cells. Appl Environ Microbiol. 1991 Sep;57(9):2651–2655. doi: 10.1128/aem.57.9.2651-2655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. A. DNA amplification by the polymerase chain reaction. Anal Chem. 1990 Jul 1;62(13):1202–1214. doi: 10.1021/ac00212a004. [DOI] [PubMed] [Google Scholar]
- Jochimsen B., Nygaard P., Vestergaard T. Location on the chromosome of Escherichia coli of genes governing purine metabolism. Adenosine deaminase (add), guanosine kinase (gsk) and hypoxanthine phosphoribosyltransferase (hpt). Mol Gen Genet. 1975 Dec 30;143(1):85–91. doi: 10.1007/BF00269424. [DOI] [PubMed] [Google Scholar]
- Mahbubani M. H., Bej A. K., Miller R., Haff L., DiCesare J., Atlas R. M. Detection of Legionella with polymerase chain reaction and gene probe methods. Mol Cell Probes. 1990 Jun;4(3):175–187. doi: 10.1016/0890-8508(90)90051-z. [DOI] [PubMed] [Google Scholar]
- Mahbubani M. H., Bej A. K., Perlin M., Schaefer F. W., 3rd, Jakubowski W., Atlas R. M. Detection of Giardia cysts by using the polymerase chain reaction and distinguishing live from dead cysts. Appl Environ Microbiol. 1991 Dec;57(12):3456–3461. doi: 10.1128/aem.57.12.3456-3461.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S. D., Josephson K. L., Bailey R. L., Gerba C. P., Pepper I. L. Rapid method for processing soil samples for polymerase chain reaction amplification of specific gene sequences. Appl Environ Microbiol. 1991 Aug;57(8):2283–2286. doi: 10.1128/aem.57.8.2283-2286.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Starnbach M. N., Falkow S., Tompkins L. S. Species-specific detection of Legionella pneumophila in water by DNA amplification and hybridization. J Clin Microbiol. 1989 Jun;27(6):1257–1261. doi: 10.1128/jcm.27.6.1257-1261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Detection of low numbers of bacterial cells in soils and sediments by polymerase chain reaction. Appl Environ Microbiol. 1992 Feb;58(2):754–757. doi: 10.1128/aem.58.2.754-757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Rapid method for direct extraction of DNA from soil and sediments. Appl Environ Microbiol. 1991 Apr;57(4):1070–1074. doi: 10.1128/aem.57.4.1070-1074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Rapid method for separation of bacterial DNA from humic substances in sediments for polymerase chain reaction. Appl Environ Microbiol. 1992 Jul;58(7):2292–2295. doi: 10.1128/aem.58.7.2292-2295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]