Abstract
Cell surface receptors that mediate endocytosis cluster into clathrin-coated pits, which pinch off to form vesicles that transport the receptors and their ligands. This multi-step process requires the coordinated action of many factors, including GTP-hydrolyzing proteins such as dynamin and regulators of actin cytoskeleton assembly. We note herein that sequestration of heterotrimeric G protein βγ subunits in intact cells strongly inhibits clathrin-coated pit-mediated endocytosis and causes rearrangement of the actin cytoskeleton. Our results suggest that cells contain a pool of free βγ and that it functions constitutively to permit endocytosis.
Clathrin-coated pits are gateways for the entry into cells of many surface-bound receptors, including those for low density lipoprotein, epidermal growth factor, and transferrin (1); G protein-coupled receptors also utilize this pathway (2–7). The formation of clathrin-coated vesicles is a complex process. Mutations in yeast that disrupt the organization of actin impair endocytosis (8, 9), and recent reports indicate that activation of Rho family GTPases, which triggers actin filament assembly at the cortex, inhibits clathrin-mediated endocytosis (10). Thus, there is an important link between actin and the function of clathrin-coated pits. Dynamin has also emerged as an important functional component of the clathrin-coated pit. The GTPase activity of dynamin seems to be required for the conversion of a coated pit at the plasma membrane into a coated vesicle (11). We demonstrated previously that the GTPase activity of dynamin is regulated by the G protein βγ subunit complex and phosphatidylinositol 4,5-bisphosphate in vitro (12, 13); this interaction was subsequently observed in intact cells (14). These experiments, together with recent reports that suggest interactions between Gβγ and Rho family GTPases (15, 16), prompted us to examine the role of heterotrimeric G proteins as regulators of receptor-mediated endocytosis. We have used recombinant adenoviruses encoding G protein subunits as well as single cell cytoplasmic microinjection techniques to demonstrate that sequestration of heterotrimeric G protein βγ subunits in intact cells strongly inhibits clathrin-coated pit-mediated endocytosis and causes rearrangement of the actin cytoskeleton.
MATERIALS AND METHODS
Construction of Recombinant Adenoviruses.
E1A-deficient recombinant adenoviruses encoding Giα1, β1, and γ2 were generated by cotransfection of pJM17- and pACCMV.pLpA-derived transfer vectors containing cDNAs encoding the respective proteins, as described by Becker et al. (17).
Endocytosis Assays.
Uptake of 125I-transferrin and calculation of internal/surface ratios of the labeled ligand were performed as described by Wiley and Cunningham (18). To quantify endocytosis of 125I-LDL, cells were first incubated with Dulbecco modified Eagle’s medium (DMEM) containing 10 μg/ml 125I-LDL at 0°C for 60 min. Cells were then washed with ice-cold DMEM and incubated at 37°C with DMEM. At the indicated times, cells were placed on ice, and surface-bound 125I-LDL was removed with 10 mM NaHepes (pH 7.4), 50 mM NaCl, and 10 mM suramin. Cells were finally solubilized with 1.0 M NaOH, and the amount of surface-bound and internalized 125I-LDL was determined by gamma counting. Endocytosis of 125I-LDL is expressed as percent ligand internalized. 125I-LDL was a gift from Joseph Goldstein and Michael Brown (Univ. of Texas Southwestern Medical Center).
Microinjection.
Cells were grown on coverslips prior to intracytoplasmic injection with G protein subunits (50 μM) together with fluorescein-conjugated dextran (2 mg/ml, 10 kDa, Molecular Probes). All protein solutions contained 2 mM NaPipes (pH 7.0), 80 mM NaCl, 20 mM KCl, 1 mM MgCl2, and 2 mM DTT. The injected volume was 100–200 fl per cell.
Quantitation of Internalized Fluorescent Ligands.
Cells were grown in DMEM supplemented with 10% fetal bovine serum. Cell Locate coverslips (Eppendorf) were used to facilitate identification. One day before experiments, the medium was replaced with dye-free DMEM containing 5% lipoprotein-deficient serum or 50 μM deferroxamine mesylate to induce expression of LDL or transferrin receptors, respectively. Two hours after injection, COS-7 cells and HEK-293 cells were incubated with 20 μg/ml FITC-transferrin (Molecular Probes), and CV1 cells were incubated with 5 μg/ml DiI-LDL (Molecular Probes) for 4 min at 37°C. Surface-bound ligand was eluted with 50 mM glycine (pH 4.8), 100 mM NaCl, and 25 mM KCl), and cells were fixed on ice in 3% paraformaldehyde and mounted in Slow Fade solution (Molecular Probes) to inhibit quenching of the FITC signal. Fluorescence images of cells were obtained with a Bio-Rad MRC 600 confocal microscope. FITC-transferrin was visualized with a 488-nm excitation filter, a 510-nm dichroic mirror, and a 515-nm long-pass emission filter. DiI-LDL was visualized with a 514-nm excitation filter, a 540-nm dichroic mirror, and a 550-nm emission filter. Each field of cells was sectioned three-dimensionally by recording images from a series of focal planes. Enough vertical sections were taken to include the top and bottom surfaces of all the cells. A two-dimensional projection was made of the three-dimensional stack of images. This image was then used to determine fluorescence intensity associated with each cell using NIH image V. 1.61 image analysis software. At least 84 cells were quantified for each protein tested. Error bars represent standard deviations.
RESULTS AND DISCUSSION
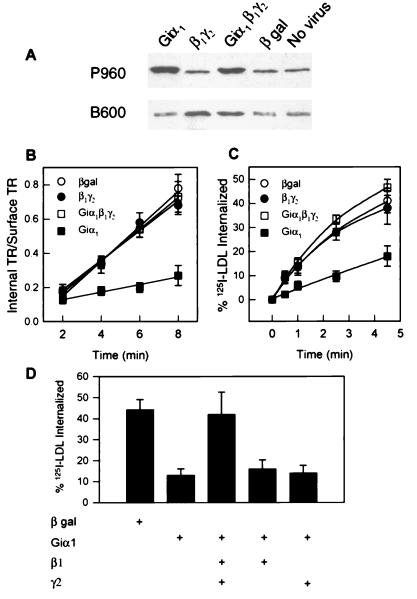
Infection with recombinant adenoviruses makes it possible to overexpress desired proteins in virtually all cells in a given culture. Cells infected with G protein-encoding viruses were examined for their capacity to internalize transferrin and LDL, two molecules that enter cells as receptor-bound complexes via clathrin-coated pits. The expression of selected G protein subunits in COS-7 cells infected with appropriate viruses was analyzed by immunoblotting (Fig. 1A). The concentration of either Giα1 or the G protein β1γ2 complex was increased significantly over levels detected with antibodies that recognize peptide epitopes common to most G protein α subunits (P960) or β subunits (B600), respectively. We then examined the initial rates of endocytosis by infected cells. Expression of Giα1 substantially inhibited endocytosis of both 125I-transferrin and 125I-LDL (Fig. 1 B and C). By contrast, endocytosis was not affected by expression of either β1γ2 or β-galactosidase. Furthermore, the inhibition of endocytosis by Giα1 was prevented by concurrent expression of β1γ2. Prevention of the effect of Giα1 required functional βγ, as expression of β1 or γ2 alone was ineffective (Fig. 1D). Endocytosis of both ligands was affected similarly, and changes in receptor number should not have affected our measurements because internalization of ligand is expressed as a fraction of the total bound.
Figure 1.
Overexpression of Giα1 inhibits receptor-mediated endocytosis. (A) Immunoblot of COS-7 cells infected with adenoviruses encoding Giα1, β1, γ2, and/or β-galactosidase. Antiserum P960 recognizes Giα1 and several other Gα proteins (31), whereas antiserum B600 recognizes β subunits (20). Hela cells (B) and CV1 cells (C and D) were grown in 35-mm 6-well culture dishes for 16 h before infection with the indicated viruses. Cell were exposed to 150 moi (multiplicity of infection) units of total virus (50 moi of virus encoding each indicated G protein subunit, the remainder encoding β-galactosidase). Cells were approximately 80% confluent at the time of infection. Cells were assayed 48 h later for uptake of 125I-transferrin (B) or 125I-LDL (C and D). Error bars represent standard deviations (n = 3).
An explanation for the inhibition caused by Giα1 is alteration of the concentrations of second messengers that might regulate endocytosis. An alternative is that expression of Giα1, present largely as the GDP-bound protein, effectively decreases the concentration of endogenous free βγ by formation of inactive heterotrimer. To distinguish between these possibilities, we injected a variety of purified wild-type or mutant G protein subunits into cells and quantified their effects on the internalization of fluorescent ligands. These proteins were purified from native tissue (Gtα), recombinant baculovirus-infected Sf9 cells (βγ), or bacteria also expressing protein N-myristoyl transferase (Giα1). These recombinant proteins thus contain the lipid modifications (prenylation of γ, myristoylation of Gα) known to influence interactions of Gα or βγ with effectors and formation of G protein heterotrimers (19, 20). We avoided the use of GTP[γS]-bound α subunits, as the free nucleotide itself has a profound inhibitory effect (Fig. 3). This is likely due to interactions between GTP[γS] and guanine nucleotide binding proteins such as dynamin, Rho, and Rac, all of which have been implicated in endocytosis (10, 11).
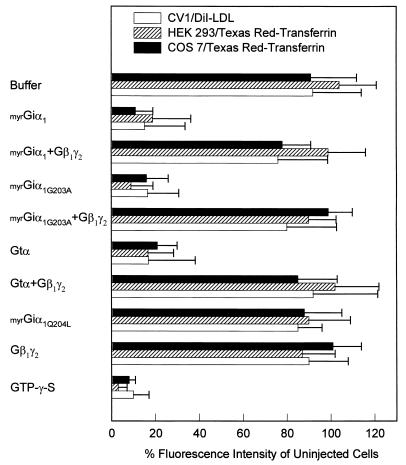
Figure 3.
Endocytosis of fluorescent ligands. Cells were injected with solutions containing 50 μM G protein subunits or 10 mM GTP[γS] as described in Fig. 2, except fluorescein-conjugated dextran was omitted. Two hours after injection, COS-7 cells and HEK-293 cells were incubated with 20 μg/ml FITC-transferrin, and CV1 cells were incubated with 5 μg/ml DiI-LDL for 4 min at 37°C. Quantitation of the internalized fluorescent ligands is described in detail under Materials and Methods.
Examples of transferrin uptake by injected cells are shown in Fig. 2. Endocytosis was further analyzed by quantitative fluorescence microscopy using three different cell lines (Fig. 3). Consistent with the results shown above, endocytosis was inhibited in cells injected with myrGiα1 (Fig. 2D); myrG203AGiα1, a mutant α subunit with high affinity for βγ, even when bound with GTP (21) (Fig. 2F); and Gtα, an α subunit whose effector is presumably confined to retinal rod cells. Furthermore, coinjection of equimolar amounts of βγ prevented the inhibition caused by injection of α subunits (Fig. 2J). Finally, injection of myrQ204LGiα1, a constitutively active form of the protein (because of mutational inactivation of intrinsic GTPase activity), did not inhibit endocytosis (Fig. 2H). These results indicate that Gα-induced inhibition of endocytosis is not due to downstream signaling but rather to sequestration of βγ. Again, endocytosis was unaltered by excess βγ itself, suggesting a constitutive role for this protein in the process and the existence of sufficient free endogenous βγ to sustain the function of clathrin-coated pits.
Figure 2.
Effects of injected G protein subunits on endocytosis. One hour after injection, cells were incubated with Texas Red-conjugated transferrin (25 μg/ml, Molecular Probes) for 5 min at 37°C. Cells were then chilled on ice, washed, and fixed with 3% paraformaldehyde. Panels A, C, E, G, and I show fluorescence of fluorescein-dextran and thus demonstrate which cell in the field was injected with buffer (A), myrGiα1 (C), myrG203AGiα1 (E), myrG203AGiα1 plus β1γ2 (G), or myrQ204LGiα1 (I). Panels B, D, F, H, and J show accumulation of Texas Red-transferrin by all cells in the same field. (Bar = 15 μm.)
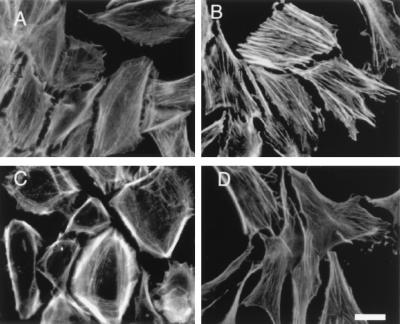
In addition to causing alterations in the rate of endocytosis, overexpression of selected G protein subunits caused dramatic changes in cell shape and the distribution of the actin cytoskeleton (Fig. 4). Gα caused cells to elongate and assemble prominent actin stress fibers along their long axis. By contrast, cells expressing excess βγ assembled actin fibers predominantly at the periphery and adopted a flattened, round shape. Expression of all three subunits resulted in yet a different morphology but little alteration in F-actin staining compared with control cells. None of the cells displayed extensive membrane ruffling.
Figure 4.
F-actin staining of CV1 cells. Cells infected for 48 h with adenoviruses encoding β-galactosidase (A), Giα1 (B), β1γ2 (C), or Giα1β1γ2 (D) were fixed and stained with Oregon green phalloidin (Molecular Probes). (Bar = 15 μm.)
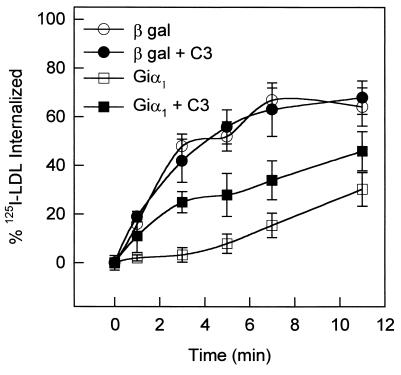
The signaling pathways that control actin fiber organization are exceedingly complex and involve several Rho family GTPases as key regulators (22, 23). In addition, two members of this family, Rac and Rho, seem to regulate endocytosis via clathrin-coated pits (10). Genetic evidence implicates the actin cytoskeleton in the endocytotic pathway in yeast (8, 9, 24); agents that specifically disrupt actin polymerization also inhibit endocytosis (25). Because the rearrangement of actin observed in cells expressing Giα1 resembles that caused by activated Rho, we sought evidence that Rho family members might be involved in the endocytotic response to G protein subunits. When cells expressing Giα1 were scrape-loaded with C3 exoenzyme from Clostridium botulinum, which specifically inactivates Rho by catalyzing its ADP ribosylation, endocytosis was partially restored (to about 50% of control, Fig. 5). This treatment did not alter the efficiency of endocytosis by control cells, indicating its specificity. Although little is known about possible direct interactions between G protein subunits and low molecular weight GTP-binding proteins, the G protein βγ subunit complex has been noted to inhibit binding of GTP[γS] to Rac and Rho but not to CDC42 (15). Thus, sequestration of free βγ might promote accumulation of GTP-bound forms of Rac and Rho, which could, in turn, inhibit endocytosis. In addition, sequestration of βγ could release dynamin from a critical regulatory influence. Interestingly, cells expressing a mutant dynamin I that blocks endocytosis also display dramatic change in morphology and rearrangement of the actin cytoskeleton (26).
Figure 5.
Giα1-mediated inhibition of endocytosis is partially reversed by C3 exoenzyme. CV1 cells infected with adenoviruses encoding Giα1 or β-galactosidase were scrape-loaded with C3 (10 μg/ml) 24 h after infection (32). 125I-LDL uptake was quantified 24 h after scrape loading, as described under Materials and Methods. Error bars represent standard deviations (n = 3).
It is likely that the pathways that control endocytosis and the assembly of the actin cytoskeleton share components, perhaps including both Rho family members and G protein subunits. This is supported by recent reports suggesting interactions between these proteins (27–29), including demonstration of signaling from G protein-coupled receptors to c-Jun kinase involving actions of βγ on a Ras and Rac1-dependent pathway (16). G protein βγ subunits participate in a large number of diverse interactions (30). The best characterized of these are with proteins that are seemingly remote to the endocytotic process, such as adenylyl cyclase, phospholipase Cβ, and ion channels. However, the recent discoveries that βγ regulates dynamin, Rho, and Rac are consistent with a more direct role in endocytosis than previously suspected. The results of sequestration of βγ with highly specific reagents suggests a hitherto unexpected requirement for the protein in receptor-mediated endocytosis and regulation of actin cytoskeleton.
Acknowledgments
We thank Dr. Joseph P. Albanesi for critical review of the manuscript and Julie Collins for excellent technical assistance. We also thank Drs. Richard J. Noel and Christopher B. Newgard (U.T. Southwestern) for pJM17, pACCMV.pLpA, and the β-galactosidase-encoding virus. This work was supported by National Institutes of Health Grants GM34497 and GM07062 and The Raymond and Ellen Willie Distinguished Chair in Molecular Neuropharmacology.
ABBREVIATIONS
- FITC
fluorescein isothiocyanate
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- LDL
low density lipoprotein
- GTP[γS]
guanosine 5′-[γ-thio]triphosphate
References
- 1.Pearse B M, Robinson M S. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- 2.von Z M, Kobilka B K. J Biol Chem. 1992;267:3530–3538. [PubMed] [Google Scholar]
- 3.Tolbert L M, Lameh J. J Biol Chem. 1996;271:17335–17342. doi: 10.1074/jbc.271.29.17335. [DOI] [PubMed] [Google Scholar]
- 4.Grady E F, Slice L W, Brant W O, Walsh J H, Payan D G, Bunnett N W. J Biol Chem. 1995;270:4603–4611. doi: 10.1074/jbc.270.9.4603. [DOI] [PubMed] [Google Scholar]
- 5.Goodman O B J, Krupnick J G, Gurevich V V, Benovic J L, Keen J H. J Biol Chem. 1997;272:15017–15022. doi: 10.1074/jbc.272.23.15017. [DOI] [PubMed] [Google Scholar]
- 6.Krupnick J G, Goodman O B J, Keen J H, Benovic J L. J Biol Chem. 1997;272:15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- 7.Goodman O B J, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 8.Munn A L, Stevenson B J, Geli M I, Riezman H. Mol Biol Cell. 1995;6:1721–1742. doi: 10.1091/mbc.6.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang H Y, Munn A, Cai M. Mol Cell Biol. 1997;17:4294–4304. doi: 10.1128/mcb.17.8.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamaze C, Chuang T H, Terlecky L J, Bokoch G M, Schmid S L. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- 11.van der Bliek A M, Meyerowitz E M. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- 12.Lin H C, Gilman A G. J Biol Chem. 1996;271:27979–27982. doi: 10.1074/jbc.271.45.27979. [DOI] [PubMed] [Google Scholar]
- 13.Lin H C, Barylko B, Achiriloaie M, Albanesi J P. J Biol Chem. 1997;272:25999–26004. doi: 10.1074/jbc.272.41.25999. [DOI] [PubMed] [Google Scholar]
- 14.Liu J P, Yajima Y, Li H, Ackland S, Akita Y, Stewart J, Kawashima S. Mol Cell Endocrinol. 1997;132:61–71. doi: 10.1016/s0303-7207(97)00120-2. [DOI] [PubMed] [Google Scholar]
- 15.Harhammer R, Gohla A, Schultz G. FEBS Lett. 1996;399:211–214. doi: 10.1016/s0014-5793(96)01327-0. [DOI] [PubMed] [Google Scholar]
- 16.Coso O A, Teramoto H, Simonds W F, Gutkind J S. J Biol Chem. 1996;271:3963–3966. doi: 10.1074/jbc.271.8.3963. [DOI] [PubMed] [Google Scholar]
- 17.Becker T C, Noel R J, Coats W S, Gomez-Foix A M, Alam T, Gerard R D, Newgard C B. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 18.Wiley H S, Cunningham D D. J Biol Chem. 1982;257:4222–4229. [PubMed] [Google Scholar]
- 19.Linder M E, Pang I H, Duronio R J, Gordon J I, Sternweis P C, Gilman A G. J Biol Chem. 1991;271:4654–4659. [PubMed] [Google Scholar]
- 20.Iniguez-Lluhi J A, Simon M I, Robishaw J D, Gilman A G. J Biol Chem. 1992;267:23409–23417. [PubMed] [Google Scholar]
- 21.Lee E, Taussig R, Gilman A G. J Biol Chem. 1992;267:1212–1218. [PubMed] [Google Scholar]
- 22.Ridley A J. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- 23.Symons M. Trends Biochem Sci. 1996;21:178–181. [PubMed] [Google Scholar]
- 24.Moreau V, Galan J M, Devilliers G, Haguenauer-Tsapis R, Winsor B. Mol Biol Cell. 1997;8:1361–1375. doi: 10.1091/mbc.8.7.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamaze C, Fujimoto L M, Yin H L, Schmid S L. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- 26.Damke H, Baba T, van der Bliek A M, Schmid S L. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fromm C, Coso O A, Montaner S, Xu N, Gutkind J S. Proc Natl Acad Sci USA. 1997;94:10098–10103. doi: 10.1073/pnas.94.19.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilk-Blaszczak M A, Singer W D, Quill T, Miller B, Frost J A, Sternweis P C, Belardetti F. J Neurosci. 1997;17:4094–4100. doi: 10.1523/JNEUROSCI.17-11-04094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buhl A M, Johnson N L, Dhanasekaran N, Johnson G L. J Biol Chem. 1995;270:24631–24634. doi: 10.1074/jbc.270.42.24631. [DOI] [PubMed] [Google Scholar]
- 30.Clapham D E, Neer E J. Nature. 1993;365:403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- 31.Mumby S M, Gilman A G. Methods Enzymol. 1991;195:215–233. doi: 10.1016/0076-6879(91)95168-j. [DOI] [PubMed] [Google Scholar]
- 32.Malcolm K C, Elliott C M, Exton J H. J Biol Chem. 1996;271:13135–13139. doi: 10.1074/jbc.271.22.13135. [DOI] [PubMed] [Google Scholar]