Abstract
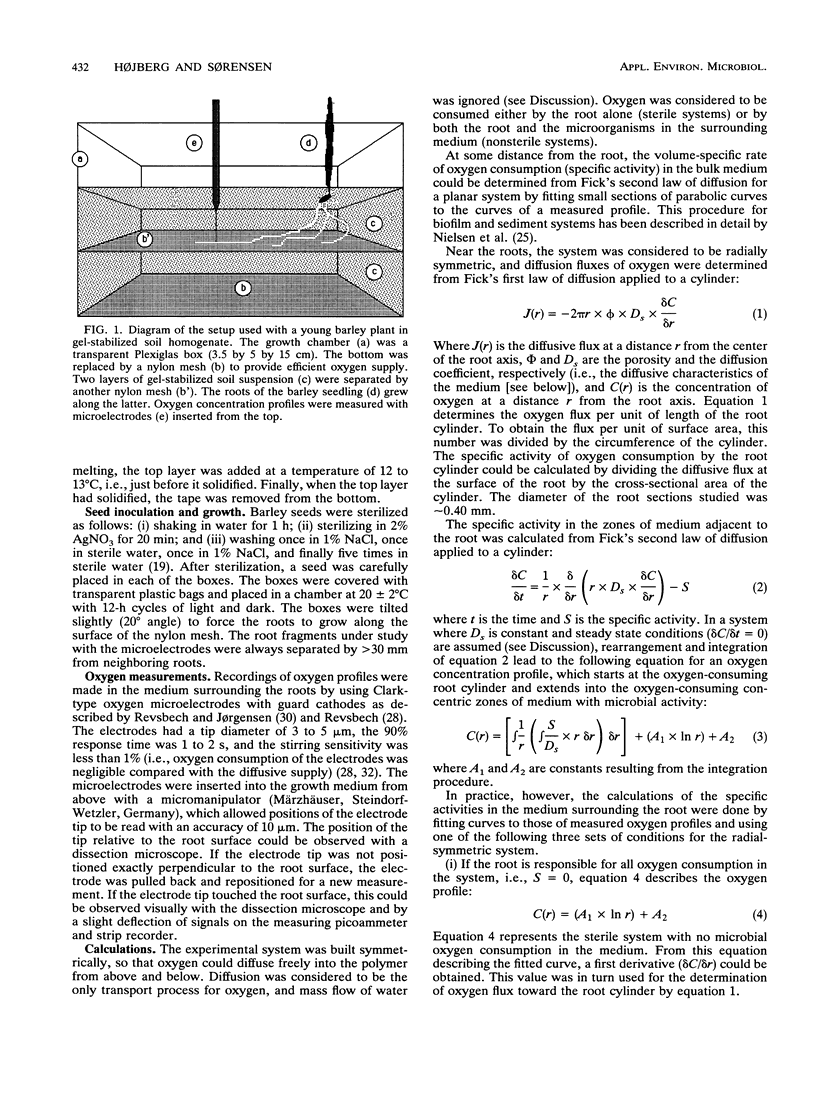
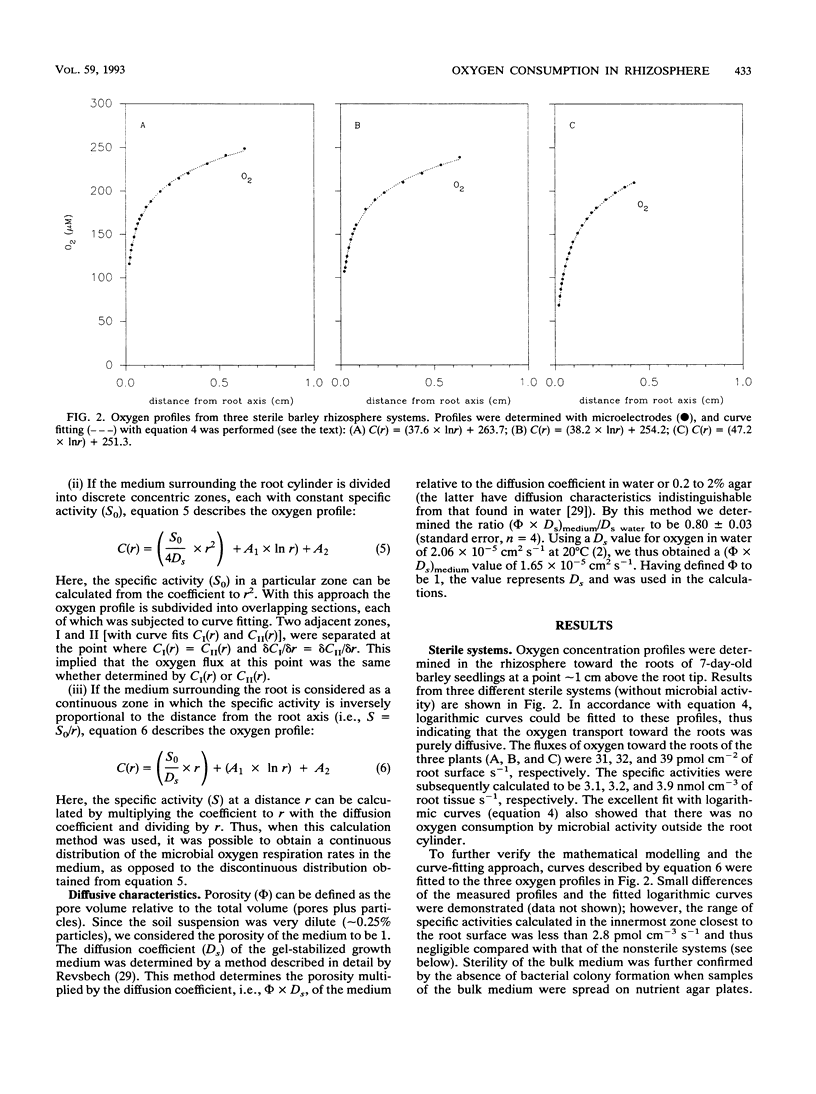
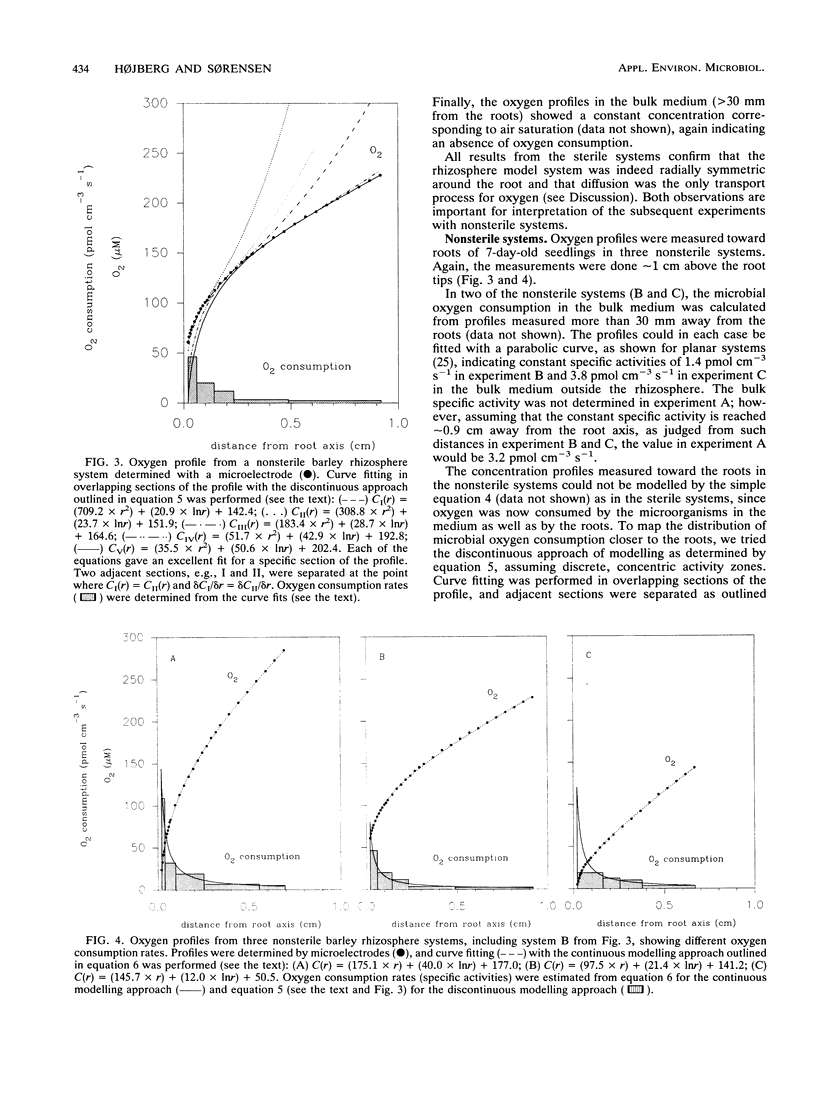
A microelectrode technique was used to map the radial distribution of oxygen concentrations and oxygen consumption rates around single roots of 7-day-old barley seedlings. The seedlings were grown in gel-stabilized medium containing a nutrient solution, a soil extract, and an inert polymer. Oxygen consumption by microbial respiration in the rhizosphere (<5 mm from the root) and in bulk medium (>30 mm from the root) was determined by using Fick's laws of diffusion and an analytical approach with curve fitting to measured microprofiles of oxygen concentration. A marked increase of microbial respiration was observed in the inner 0- to 3-mm-thick, concentric zone around the root (rhizosphere). The volume-specific oxygen consumption rate (specific activity) was thus 30 to 60 times higher in the innermost 0 to 0.01 mm (rhizoplane) than in the bulk medium. The oxygen consumption rate in the root tissue was in turn 10 to 30 times higher than that in the rhizoplane. Both microbial respiration and oxygen uptake by the root varied between different roots. This was probably due to a between-root variation of the exudation rate for easily degradable carbon compounds supporting the microbial oxygen consumption.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binnerup S. J., Sørensen J. Nitrate and nitrite microgradients in barley rhizosphere as detected by a highly sensitive denitrification bioassay. Appl Environ Microbiol. 1992 Aug;58(8):2375–2380. doi: 10.1128/aem.58.8.2375-2380.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes M. T. Aquatic nitrogen transformations at low oxygen concentrations. Appl Environ Microbiol. 1988 Jan;54(1):172–175. doi: 10.1128/aem.54.1.172-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goreau T. J., Kaplan W. A., Wofsy S. C., McElroy M. B., Valois F. W., Watson S. W. Production of NO(2) and N(2)O by Nitrifying Bacteria at Reduced Concentrations of Oxygen. Appl Environ Microbiol. 1980 Sep;40(3):526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen G. H., Bloom A. J., Spanswick R. M. Measurement of net fluxes of ammonium and nitrate at the surface of barley roots using ion-selective microelectrodes. Plant Physiol. 1990 May;93(1):271–280. doi: 10.1104/pp.93.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. Denitrification. Microbiol Rev. 1982 Mar;46(1):43–70. doi: 10.1128/mr.46.1.43-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poth M., Focht D. D. N Kinetic Analysis of N(2)O Production by Nitrosomonas europaea: an Examination of Nitrifier Denitrification. Appl Environ Microbiol. 1985 May;49(5):1134–1141. doi: 10.1128/aem.49.5.1134-1141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsbech N. P., Ward D. M. Oxygen Microelectrode That Is Insensitive to Medium Chemical Composition: Use in an Acid Microbial Mat Dominated by Cyanidium caldarium. Appl Environ Microbiol. 1983 Mar;45(3):755–759. doi: 10.1128/aem.45.3.755-759.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



