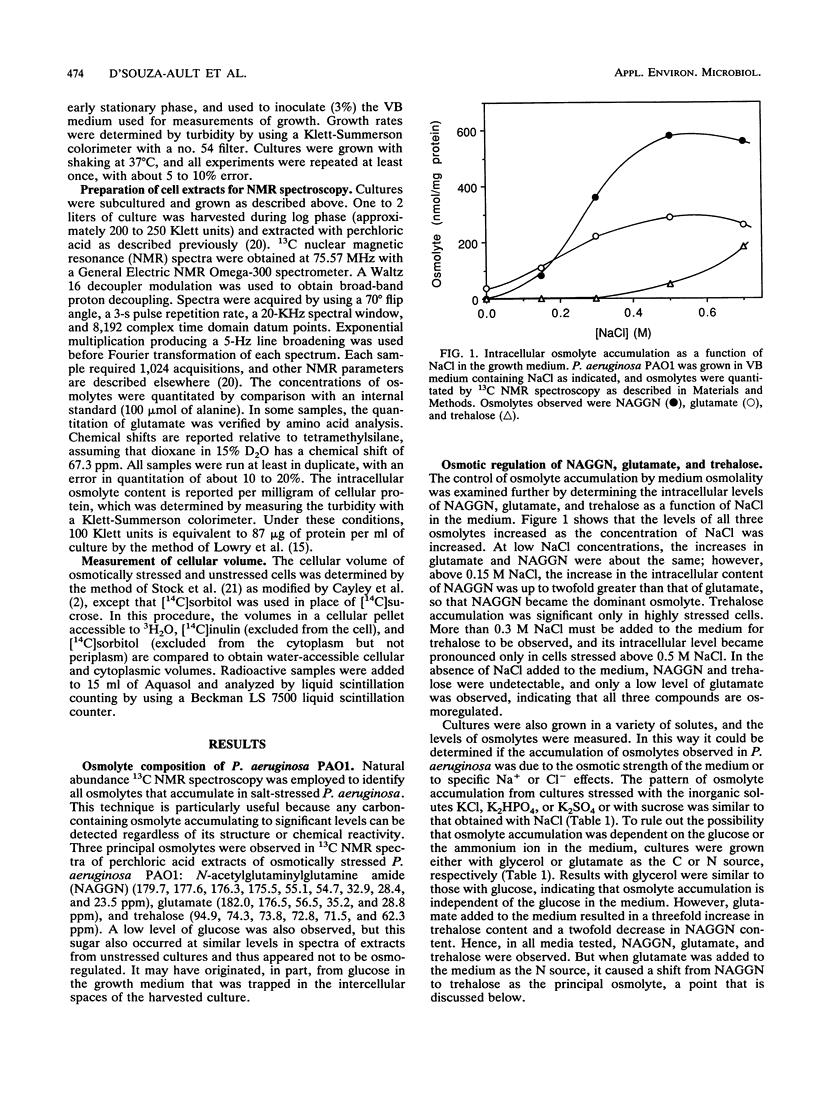
Abstract
The mechanism of osmotic stress adaptation in Pseudomonas aeruginosa PAO1 was investigated. By using natural abundance 13C nuclear magnetic resonance spectroscopy, osmotically stressed cultures were found to accumulate glutamate, trehalose, and N-acetylglutaminylglutamine amide, an unusual dipeptide previously reported only in osmotically stressed Rhizobium meliloti and Pseudomonas fluorescens. The intracellular levels of these osmolytes were dependent on the chemical composition and the osmolality of the growth medium. It was also demonstrated that glycine betaine, a powerful osmotic stress protectant, participates in osmoregulation in this organism. When glycine betaine or its precursors, phosphorylcholine or choline, were added to the growth medium, growth rates of cultures in 0.7 M NaCl were increased more than threefold. Furthermore, enhancement of growth could be observed with as little as 10 microM glycine betaine or precursor added to the medium. Finally, the mechanism of osmotic stress adaptation of two clinical isolates of P. aeruginosa was found to be nearly identical to that of the laboratory strain PAO1 in all aspects studied.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry A., DeVault J. D., Chakrabarty A. M. High osmolarity is a signal for enhanced algD transcription in mucoid and nonmucoid Pseudomonas aeruginosa strains. J Bacteriol. 1989 May;171(5):2312–2317. doi: 10.1128/jb.171.5.2312-2317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERNICK W. S., BARBERO G. J. Composition of tracheobronchial secretions in cystic fibrosis of the pancreas and bronchiectasis. Pediatrics. 1959 Nov;24:739–745. [PubMed] [Google Scholar]
- Cayley S., Lewis B. A., Guttman H. J., Record M. T., Jr Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J Mol Biol. 1991 Nov 20;222(2):281–300. doi: 10.1016/0022-2836(91)90212-o. [DOI] [PubMed] [Google Scholar]
- Csonka L. N., Hanson A. D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Govan J. R., Konyecsni W. M., Martin D. W. Mucoid Pseudomonas aeruginosa in cystic fibrosis: mutations in the muc loci affect transcription of the algR and algD genes in response to environmental stimuli. Mol Microbiol. 1990 Feb;4(2):189–196. doi: 10.1111/j.1365-2958.1990.tb00586.x. [DOI] [PubMed] [Google Scholar]
- Eshoo M. W. lac fusion analysis of the bet genes of Escherichia coli: regulation by osmolarity, temperature, oxygen, choline, and glycine betaine. J Bacteriol. 1988 Nov;170(11):5208–5215. doi: 10.1128/jb.170.11.5208-5215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan P. H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991 Jan;4(1):35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Berka R. M., Vasil M. L. A Pseudomonas aeruginosa mutant non-derepressible for orthophosphate-regulated proteins. J Bacteriol. 1981 Aug;147(2):675–678. doi: 10.1128/jb.147.2.675-678.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge-Aronis R., Klein W., Lange R., Rimmele M., Boos W. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J Bacteriol. 1991 Dec;173(24):7918–7924. doi: 10.1128/jb.173.24.7918-7924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortstee G. J. The aerobic decomposition of choline by microorganisms. I. The ability of aerobic organisms, particularly coryneform bacteria, to utilize choline as the sole carbon and nitrogen source. Arch Mikrobiol. 1970;71(3):235–244. [PubMed] [Google Scholar]
- Krieg D. P., Bass J. A., Mattingly S. J. Phosphorylcholine stimulates capsule formation of phosphate-limited mucoid Pseudomonas aeruginosa. Infect Immun. 1988 Apr;56(4):864–873. doi: 10.1128/iai.56.4.864-873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen P. I., Sydnes L. K., Landfald B., Strøm A. R. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol. 1987 Feb;147(1):1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- MATTHEWS L. W., SPECTOR S., LEMM J., POTTER J. L. STUDIES ON PULMONARY SECRETIONS. I. THE OVER-ALL CHEMICAL COMPOSITION OF PULMONARY SECRETIONS FROM PATIENTS WITH CYSTIC FIBROSIS, BRONCHIECTASIS, AND LARYNGECTOMY. Am Rev Respir Dis. 1963 Aug;88:199–204. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- Madkour M. A., Smith L. T., Smith G. M. Preferential osmolyte accumulation: a mechanism of osmotic stress adaptation in diazotrophic bacteria. Appl Environ Microbiol. 1990 Sep;56(9):2876–2881. doi: 10.1128/aem.56.9.2876-2881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measures J. C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature. 1975 Oct 2;257(5525):398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- Smith L. T., Smith G. M. An osmoregulated dipeptide in stressed Rhizobium meliloti. J Bacteriol. 1989 Sep;171(9):4714–4717. doi: 10.1128/jb.171.9.4714-4717.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]



