Abstract
The penultimate step in the aflatoxin biosynthetic pathway of the filamentous fungi Aspergillus flavus and A. parasiticus involves conversion of sterigmatocystin to O-methylsterigmatocystin. An S-adenosylmethionine-dependent methyltransferase that catalyzes this reaction was purified to homogeneity (> 90%) from 78-h-old mycelia of A. parasiticus SRRC 163. Purification of this soluble enzyme was carried out by five soft-gel chromatographic steps: cell debris remover treatment, QMA ACELL chromatography, hydroxylapatite-Ultrogel chromatography, DEAE-Spherodex chromatography, and Octyl Avidgel chromatography, followed by MA7Q high-performance liquid chromatography. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the protein peak from this step on silver staining identified a single band of approximately 40 kDa. This purified protein was distinct from the dimeric 168-kDa methyltransferase purified from the same fungal strain under identical growth conditions (D. Bhatnagar, A. H. J. Ullah, and T. E. Cleveland, Prep. Biochem. 18:321-349, 1988). The chromatographic behavior and N-terminal sequence of the 40-kDa enzyme were also distinct from those of the 168-kDa methyltransferase. The molar extinction coefficient of the 40-kDa enzyme at 278 nm was estimated to be 4.7 x 10(4) M-1 cm-1 in 50 mM potassium phosphate buffer (pH 7.5).
Full text
PDF
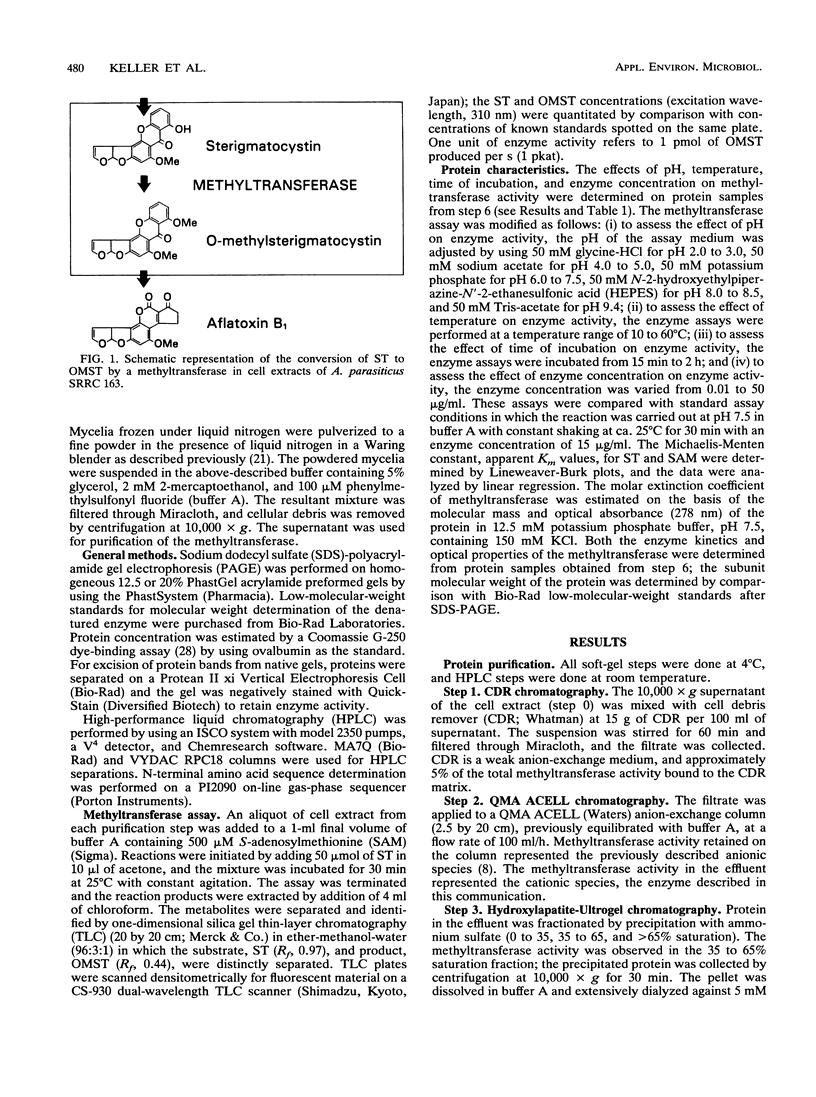
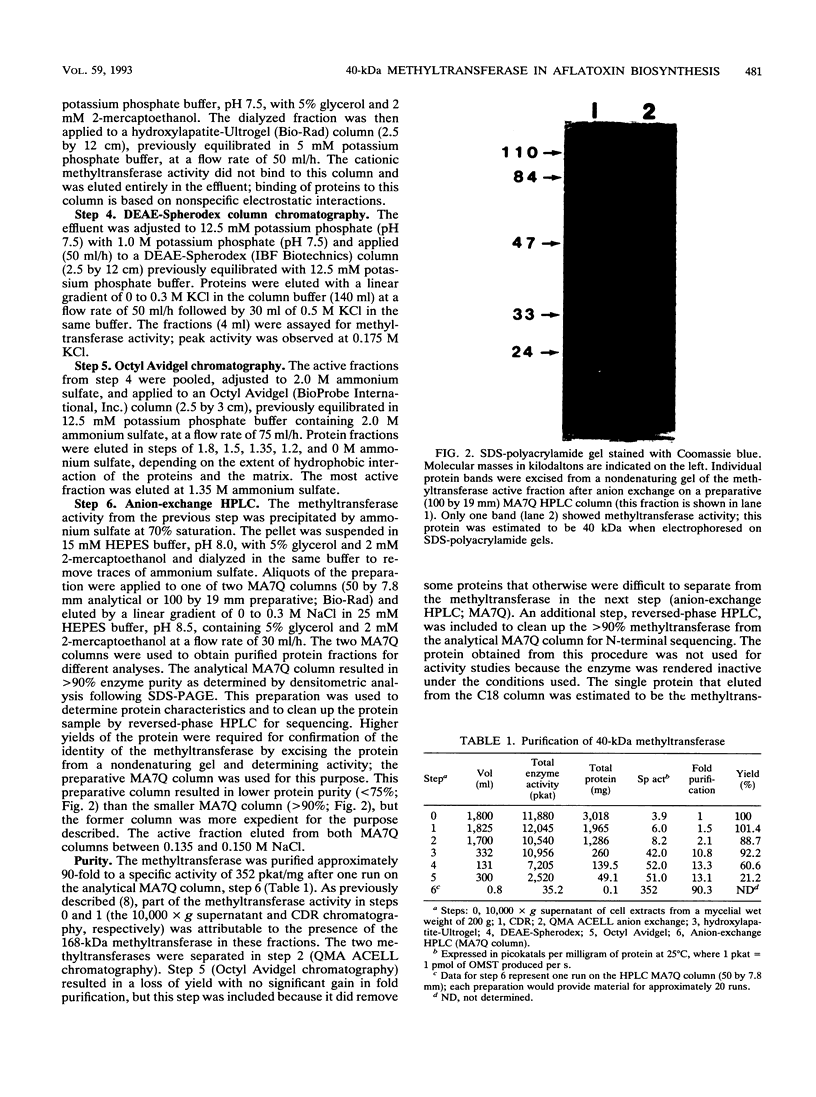
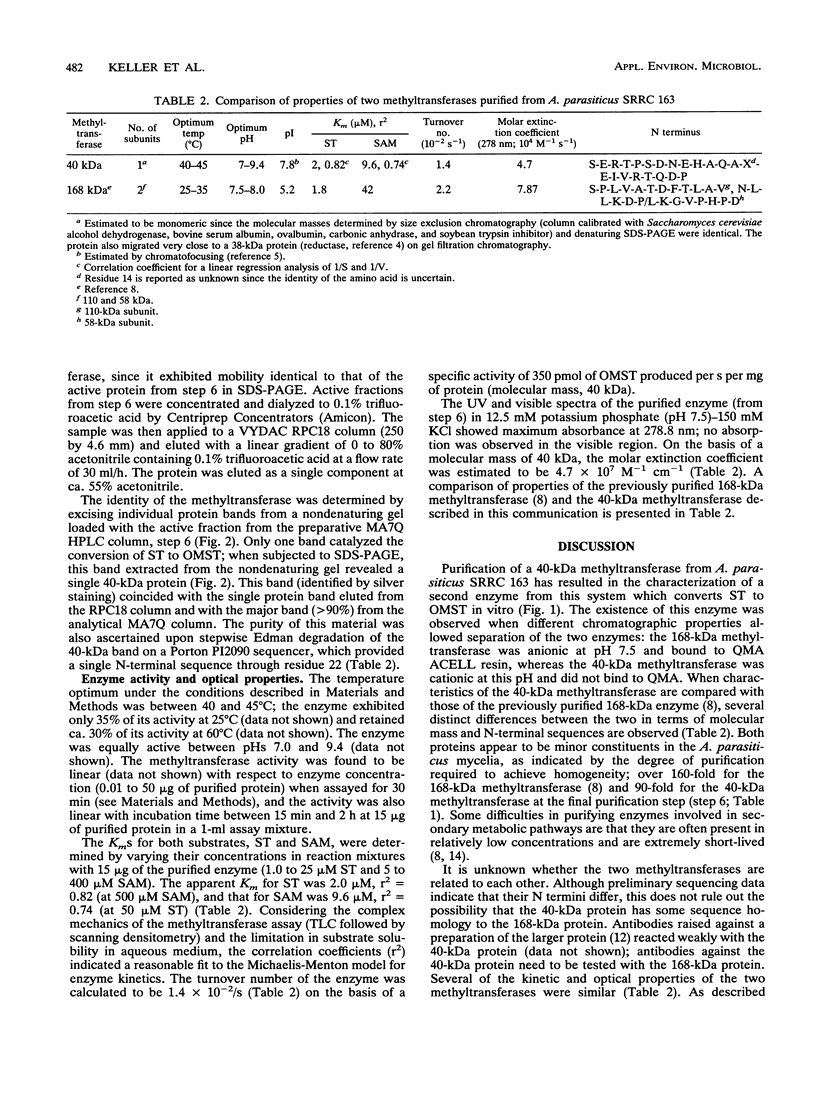


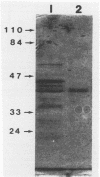
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADYE J., MATELES R. I. INCORPORATION OF LABELLED COMPOUNDS INTO AFLATOXINS. Biochim Biophys Acta. 1964 May 11;86:418–420. doi: 10.1016/0304-4165(64)90077-7. [DOI] [PubMed] [Google Scholar]
- Anderson J. A., Chung C. H., Cho S. H. Versicolorin A hemiacetal, hydroxydihydrosterigmatocystin, and aflatoxin G2 alpha reductase activity in extracts from Aspergillus parasiticus. Mycopathologia. 1990 Jul;111(1):39–45. doi: 10.1007/BF02277300. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Lee L. S., Shoss S. M., Boudreaux G. H. Identification of averantin as an aflatoxin B1 precursor: placement in the biosynthetic pathway. Appl Environ Microbiol. 1980 Apr;39(4):835–839. doi: 10.1128/aem.39.4.835-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar D., Cleveland T. E., Kingston D. G. Enzymological evidence for separate pathways for aflatoxin B1 and B2 biosynthesis. Biochemistry. 1991 Apr 30;30(17):4343–4350. doi: 10.1021/bi00231a033. [DOI] [PubMed] [Google Scholar]
- Bhatnagar D., McCormick S. P., Lee L. S., Hill R. A. Identification of O-methylsterigmatocystin as an aflatoxin B1 and G1 precursor in Aspergillus parasiticus. Appl Environ Microbiol. 1987 May;53(5):1028–1033. doi: 10.1128/aem.53.5.1028-1033.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar D., Ullah A. H., Cleveland T. E. Purification and characterization of a methyltransferase from Aspergillus parasiticus SRRC 163 involved in aflatoxin biosynthetic pathway. Prep Biochem. 1988;18(3):321–349. doi: 10.1080/00327488808062532. [DOI] [PubMed] [Google Scholar]
- Chuturgoon A. A., Dutton M. F., Berry R. K. The preparation of an enzyme associated with aflatoxin biosynthesis by affinity chromatography. Biochem Biophys Res Commun. 1990 Jan 15;166(1):38–42. doi: 10.1016/0006-291x(90)91908-b. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E., Bhatnagar D. Evidence for de novo synthesis of an aflatoxin pathway methyltransferase near the cessation of active growth and the onset of aflatoxin biosynthesis in Aspergillus parasiticus mycelia. Can J Microbiol. 1990 Jan;36(1):1–5. doi: 10.1139/m90-001. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E., Bhatnagar D., Foell C. J., McCormick S. P. Conversion of a new metabolite to aflatoxin B2 by Aspergillus parasiticus. Appl Environ Microbiol. 1987 Dec;53(12):2804–2807. doi: 10.1128/aem.53.12.2804-2807.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland T. E., Bhatnagar D. Individual reaction requirements of two enzyme activities, isolated from Aspergillus parasiticus, which together catalyze conversion of sterigmatocystin to aflatoxin B1. Can J Microbiol. 1987 Dec;33(12):1108–1112. doi: 10.1139/m87-193. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E., Lax A. R., Lee L. S., Bhatnagar D. Appearance of enzyme activities catalyzing conversion of sterigmatocystin to aflatoxin B1 in late-growth-phase Aspergillus parasiticus cultures. Appl Environ Microbiol. 1987 Jul;53(7):1711–1713. doi: 10.1128/aem.53.7.1711-1713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R. J. Indirect costs: FASEB looks for the answers. FASEB J. 1991 Sep;5(12):2623–2623. doi: 10.1096/fasebj.5.12.1916085. [DOI] [PubMed] [Google Scholar]
- Dutton M. F. Enzymes and aflatoxin biosynthesis. Microbiol Rev. 1988 Jun;52(2):274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh D. P., Lin M. T., Yao R. C. Conversion of sterigmatocystin to aflatoxin B 1 by Aspergillus parasiticus. Biochem Biophys Res Commun. 1973 Jun 8;52(3):992–997. doi: 10.1016/0006-291x(73)91035-8. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Lin M. T., Yao R. C., Singh R. Biosynthesis of aflatoxin. Conversion of norsolorinic acid and other hypothetical intermediates into aflatoxin B1. J Agric Food Chem. 1976 Nov-Dec;24(6):1170–1174. doi: 10.1021/jf60208a018. [DOI] [PubMed] [Google Scholar]
- Hsieh D. P., Wan C. C., Billington J. A. A versiconal hemiacetal acetate converting enzyme in aflatoxin biosynthesis. Mycopathologia. 1989 Sep;107(2-3):121–126. doi: 10.1007/BF00707548. [DOI] [PubMed] [Google Scholar]
- Ingrosso D., Fowler A. V., Bleibaum J., Clarke S. Sequence of the D-aspartyl/L-isoaspartyl protein methyltransferase from human erythrocytes. Common sequence motifs for protein, DNA, RNA, and small molecule S-adenosylmethionine-dependent methyltransferases. J Biol Chem. 1989 Nov 25;264(33):20131–20139. [PubMed] [Google Scholar]
- Lauster R. Evolution of type II DNA methyltransferases. A gene duplication model. J Mol Biol. 1989 Mar 20;206(2):313–321. doi: 10.1016/0022-2836(89)90481-6. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Bennett J. W., Cucullu A. F., Ory R. L. Biosynthesis of aflatoxin B1. Conversion of versicolorin A to aflatoxin B1 by Aspergillus parasiticus. J Agric Food Chem. 1976 Nov-Dec;24(6):1167–1170. doi: 10.1021/jf60208a017. [DOI] [PubMed] [Google Scholar]
- Lin M. T., Hsieh D. P., Yao R. C., Donkersloot J. A. Conversion of averufin into aflatoxins by Aspergillus parasiticus. Biochemistry. 1973 Dec 4;12(25):5167–5171. doi: 10.1021/bi00749a023. [DOI] [PubMed] [Google Scholar]
- Martin C. N., Garner R. C. Aflatoxin B -oxide generated by chemical or enzymic oxidation of aflatoxin B1 causes guanine substitution in nucleic acids. Nature. 1977 Jun 30;267(5614):863–865. doi: 10.1038/267863a0. [DOI] [PubMed] [Google Scholar]
- McCormick S. P., Bhatnagar D., Lee L. S. Averufanin is an aflatoxin B1 precursor between averantin and averufin in the biosynthetic pathway. Appl Environ Microbiol. 1987 Jan;53(1):14–16. doi: 10.1128/aem.53.1.14-16.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon G., Hanson L., Lee J. J., Wogan G. N. Identification of an activated c-Ki-ras oncogene in rat liver tumors induced by aflatoxin B1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9418–9422. doi: 10.1073/pnas.83.24.9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder H. W., Cole R. J., Grigsby R. D., Hein H., Jr Inhibition of aflatoxin production and tentative identification of an aflatoxin intermediate "versiconal acetate" from treatment with dichlorvos. Appl Microbiol. 1974 Feb;27(2):394–399. doi: 10.1128/am.27.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Annau T. M., Chandrasegaran S. Finding sequence motifs in groups of functionally related proteins. Proc Natl Acad Sci U S A. 1990 Jan;87(2):826–830. doi: 10.1073/pnas.87.2.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Ando Y., Hamasaki T. A metabolic grid among versiconal hemiacetal acetate, versiconol acetate, versiconol and versiconal during aflatoxin biosynthesis. J Gen Microbiol. 1991 Oct;137(10):2469–2475. doi: 10.1099/00221287-137-10-2469. [DOI] [PubMed] [Google Scholar]
- Yabe K., Ando Y., Hamasaki T. Biosynthetic relationship among aflatoxins B1, B2, G1, and G2. Appl Environ Microbiol. 1988 Aug;54(8):2101–2106. doi: 10.1128/aem.54.8.2101-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Ando Y., Hashimoto J., Hamasaki T. Two distinct O-methyltransferases in aflatoxin biosynthesis. Appl Environ Microbiol. 1989 Sep;55(9):2172–2177. doi: 10.1128/aem.55.9.2172-2177.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Nakamura Y., Nakajima H., Ando Y., Hamasaki T. Enzymatic conversion of norsolorinic acid to averufin in aflatoxin biosynthesis. Appl Environ Microbiol. 1991 May;57(5):1340–1345. doi: 10.1128/aem.57.5.1340-1345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]