Abstract
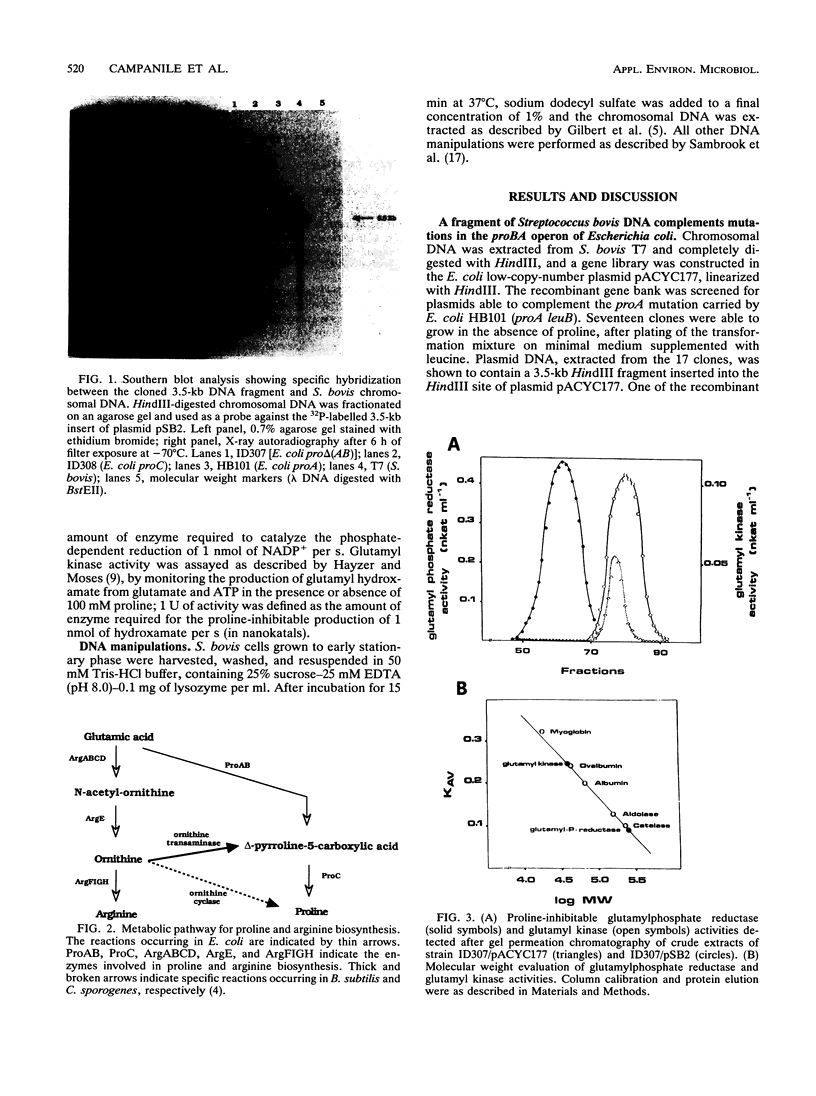
A genomic DNA library of the rumen bacterium Streptococcus bovis was constructed in Escherichia coli, and recombinant plasmids able to complement proA and proB mutations of the host were found. Southern hybridization and restriction analysis showed that a 3.5-kb fragment of S. bovis DNA contained two genes, organized in an operon and coding for enzymes functionally similar to the glutamyl phosphate reductase-glutamyl kinase enzyme complex that in E. coli catalyzes the first step of proline biosynthesis. Complementation of the E. coli mutations was observed with the fragment inserted in both orientations, which suggested that the S. bovis proBA operon was transcribed from its own promoter. Genetic and biochemical data suggested that the proline biosynthetic pathway of S. bovis is similar to the one previously characterized for E. coli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark R. G., Hu Y. J., Hynes M. F., Salmon R. K., Cheng K. J. Cloning and expression of an amylase gene from Streptococcus bovis in Escherichia coli. Arch Microbiol. 1992;157(3):201–204. doi: 10.1007/BF00245149. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fons M., Cami B., Patte J. C., Chippaux M. Cloning in Escherichia coli of genes involved in the synthesis of proline and leucine in Desulfovibrio desulfuricans Norway. Mol Gen Genet. 1987 Jan;206(1):141–143. doi: 10.1007/BF00326549. [DOI] [PubMed] [Google Scholar]
- Gilbert H. J., Clarke I. N., Gibson R. K., Stephenson J. R., Tully M. Molecular cloning of the phenylalanine ammonia lyase gene from Rhodosporidium toruloides in Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):314–320. doi: 10.1128/jb.161.1.314-320.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayzer D. J., Moses V. The enzymes of proline biosynthesis in Escherichia coli. Their molecular weights and the problem of enzyme aggregation. Biochem J. 1978 Jul 1;173(1):219–228. doi: 10.1042/bj1730219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayzer D. J. Sub-cloning of the wild-type proAB region of the Escherichia coli genome. J Gen Microbiol. 1983 Oct;129(10):3215–3225. doi: 10.1099/00221287-129-10-3215. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Whitehead T. R. Introduction of Tn916 and pAM beta 1 into Streptococcus bovis JB1 by conjugation. Appl Environ Microbiol. 1991 Sep;57(9):2710–2713. doi: 10.1128/aem.57.9.2710-2713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna R. V., Leisinger T. Biosynthesis of proline in Pseudomonas aeruginosa. Partial purification and characterization of gamma-glutamyl kinase. Biochem J. 1979 Jul 1;181(1):215–222. doi: 10.1042/bj1810215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori K., Suzuki S., Imai Y., Komatsubara S. Analysis of the Serratia marcescens proBA operon and feedback control of proline biosynthesis. J Gen Microbiol. 1991 Mar;137(3):509–517. doi: 10.1099/00221287-137-3-509. [DOI] [PubMed] [Google Scholar]
- Russell J. B. Intracellular pH of acid-tolerant ruminal bacteria. Appl Environ Microbiol. 1991 Nov;57(11):3383–3384. doi: 10.1128/aem.57.11.3383-3384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Robinson P. H. Compositions and characteristics of strains of Streptococcus bovis. J Dairy Sci. 1984 Jul;67(7):1525–1531. doi: 10.3168/jds.S0022-0302(84)81471-X. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J., Driessen A. J., Konings W. N. Sodium-dependent transport of neutral amino acids by whole cells and membrane vesicles of Streptococcus bovis, a ruminal bacterium. J Bacteriol. 1988 Aug;170(8):3531–3536. doi: 10.1128/jb.170.8.3531-3536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Wilson D. B. Potential opportunities and problems for genetically altered rumen microorganisms. J Nutr. 1988 Feb;118(2):271–279. doi: 10.1093/jn/118.2.271. [DOI] [PubMed] [Google Scholar]
- Slyter L. L. Influence of acidosis on rumen function. J Anim Sci. 1976 Oct;43(4):910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- Viviani R. Biosynthesis of essential amino acids in ruminal bacteria. Folia Vet Lat. 1976 Apr-Jun;6(2):120–174. [PubMed] [Google Scholar]
- Williams I., Frank L. Improved chemical synthesis and enzymatic assay of delta-1-pyrroline-5-carboxylic acid. Anal Biochem. 1975 Mar;64(1):85–97. doi: 10.1016/0003-2697(75)90408-x. [DOI] [PubMed] [Google Scholar]