Abstract
Epidemiological studies of shigellosis in Bangladesh have demonstrated that surface-water sources can act as foci of infection. Studies of laboratory microcosms have shown that shigellae become nonculturable but remain viable when exposed to environmental samples of water. The present study was carried out to detect viable but nonculturable Shigella dysenteriae 1 from laboratory microcosms by the polymerase chain reaction and the fluorescent-antibody techniques. S. dysenteriae 1 was inoculated into laboratory microcosms consisting of water samples collected from ponds, lakes, rivers, and drains in Bangladesh. The survival of S. dysenteriae in microcosms was assessed by viable counting on MacConkey agar. After 2 to 3 weeks, S. dysenteriae 1 became nonculturable but remained viable. After 6 weeks, this nonculturable but viable S. dysenteriae 1 was detected by both the polymerase chain reaction and the fluorescent-antibody methods. The viable but nonculturable state of S. dysenteriae 1 demonstrated in this study may be important for understanding the epidemiology of shigellosis.
Full text
PDF



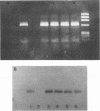
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buysse J. M., Stover C. K., Oaks E. V., Venkatesan M., Kopecko D. J. Molecular cloning of invasion plasmid antigen (ipa) genes from Shigella flexneri: analysis of ipa gene products and genetic mapping. J Bacteriol. 1987 Jun;169(6):2561–2569. doi: 10.1128/jb.169.6.2561-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chosa H., Makino S., Sasakawa C., Okada N., Yamada M., Komatsu K., Suk J. S., Yoshikawa M. Loss of virulence in Shigella strains preserved in culture collections due to molecular alteration of the invasion plasmid. Microb Pathog. 1989 May;6(5):337–342. doi: 10.1016/0882-4010(89)90075-2. [DOI] [PubMed] [Google Scholar]
- Echeverria P., Sethabutr O., Serichantalergs O., Lexomboon U., Tamura K. Shigella and enteroinvasive Escherichia coli infections in households of children with dysentery in Bangkok. J Infect Dis. 1992 Jan;165(1):144–147. doi: 10.1093/infdis/165.1.144. [DOI] [PubMed] [Google Scholar]
- Hartman A. B., Venkatesan M., Oaks E. V., Buysse J. M. Sequence and molecular characterization of a multicopy invasion plasmid antigen gene, ipaH, of Shigella flexneri. J Bacteriol. 1990 Apr;172(4):1905–1915. doi: 10.1128/jb.172.4.1905-1915.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoben H. J., Somasegaran P. Comparison of the Pour, Spread, and Drop Plate Methods for Enumeration of Rhizobium spp. in Inoculants Made from Presterilized Peat. Appl Environ Microbiol. 1982 Nov;44(5):1246–1247. doi: 10.1128/aem.44.5.1246-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M. A., Albert M. J., Hasan K. Z. Epidemiology of shigellosis in Teknaf, a coastal area of Bangladesh: a 10-year survey. Epidemiol Infect. 1990 Aug;105(1):41–49. doi: 10.1017/s0950268800047622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. S., Alam M. J., Khan S. I. Distribution of Plesiomonas shigelloides in various components of pond ecosystems in Dhaka, Bangladesh. Microbiol Immunol. 1991;35(11):927–932. doi: 10.1111/j.1348-0421.1991.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Islam M. S., Drasar B. S., Bradley D. J. Long-term persistence of toxigenic Vibrio cholerae 01 in the mucilaginous sheath of a blue-green alga, Anabaena variabilis. J Trop Med Hyg. 1990 Apr;93(2):133–139. [PubMed] [Google Scholar]
- Jablonski E., Moomaw E. W., Tullis R. H., Ruth J. L. Preparation of oligodeoxynucleotide-alkaline phosphatase conjugates and their use as hybridization probes. Nucleic Acids Res. 1986 Aug 11;14(15):6115–6128. doi: 10.1093/nar/14.15.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. M., Sutcliffe E. M., Curry A. Recovery of viable but non-culturable Campylobacter jejuni. J Gen Microbiol. 1991 Oct;137(10):2477–2482. doi: 10.1099/00221287-137-10-2477. [DOI] [PubMed] [Google Scholar]
- Khan M., Curlin G. T., Huq I. Epidemiology of Shigella dysenteriae, type 1 infections, in Dacca urban area. Trop Geogr Med. 1979 Jun;31(2):213–223. [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Lampel K. A., Jagow J. A., Trucksess M., Hill W. E. Polymerase chain reaction for detection of invasive Shigella flexneri in food. Appl Environ Microbiol. 1990 Jun;56(6):1536–1540. doi: 10.1128/aem.56.6.1536-1540.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., DuPont H. L., Formal S. B., Hornick R. B., Takeuchi A., Gangarosa E. J., Snyder M. J., Libonati J. P. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973 Mar;127(3):261–270. doi: 10.1093/infdis/127.3.261. [DOI] [PubMed] [Google Scholar]
- Nilsson L., Oliver J. D., Kjelleberg S. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J Bacteriol. 1991 Aug;173(16):5054–5059. doi: 10.1128/jb.173.16.5054-5059.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahaman M. M., Khan M. M., Aziz K. M., Islam M. S., Kibriya A. K. An outbreak of dysentery caused by Shigella dysenteriae type 1 on a coral island in the Bay of Bengal. J Infect Dis. 1975 Jul;132(1):15–19. doi: 10.1093/infdis/132.1.15. [DOI] [PubMed] [Google Scholar]
- Rollins D. M., Colwell R. R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986 Sep;52(3):531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. L., Hazlet K. K., Schaefer J., Wells J. G., Pruneda R. C. Shigellosis from swimming. JAMA. 1976 Oct 18;236(16):1849–1852. [PubMed] [Google Scholar]
- Roszak D. B., Grimes D. J., Colwell R. R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984 Mar;30(3):334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Hartman A. B. Sequence variation in two ipaH genes of Shigella flexneri 5 and homology to the LRG-like family of proteins. Mol Microbiol. 1991 Oct;5(10):2435–2445. doi: 10.1111/j.1365-2958.1991.tb02089.x. [DOI] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Kopecko D. J. Use of Shigella flexneri ipaC and ipaH gene sequences for the general identification of Shigella spp. and enteroinvasive Escherichia coli. J Clin Microbiol. 1989 Dec;27(12):2687–2691. doi: 10.1128/jcm.27.12.2687-2691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M., Buysse J. M., Vandendries E., Kopecko D. J. Development and testing of invasion-associated DNA probes for detection of Shigella spp. and enteroinvasive Escherichia coli. J Clin Microbiol. 1988 Feb;26(2):261–266. doi: 10.1128/jcm.26.2.261-266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. K., Morris J. G., Jr, Small P. L., Sethabutr O., Toledo M. R., Trabulsi L., Kaper J. B. Comparison of DNA probes and the Sereny test for identification of invasive Shigella and Escherichia coli strains. J Clin Microbiol. 1986 Sep;24(3):498–500. doi: 10.1128/jcm.24.3.498-500.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]