Abstract
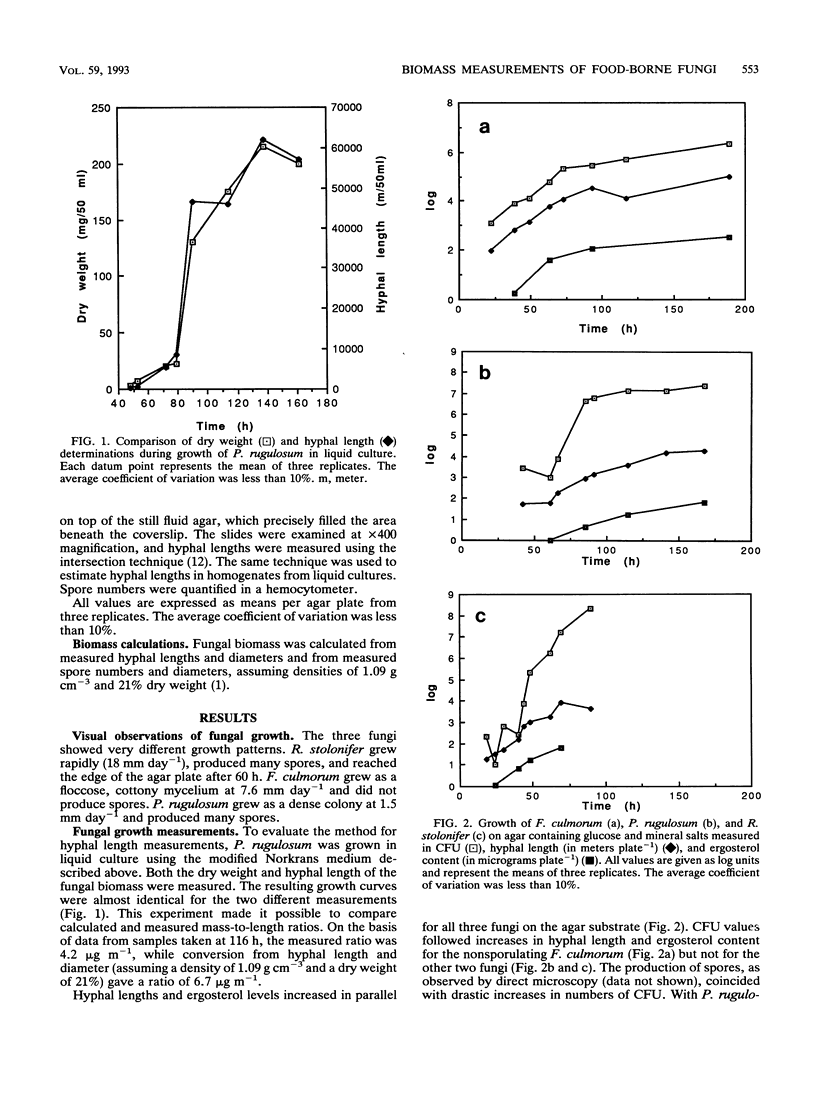
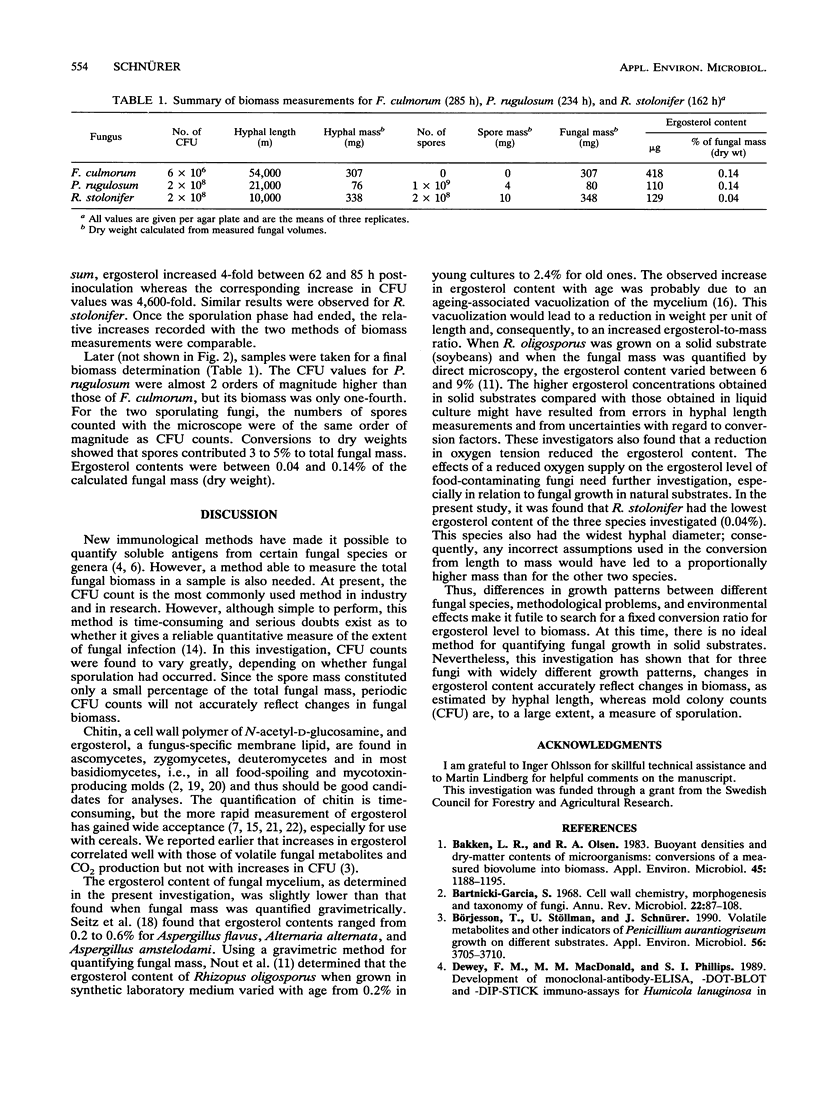
To evaluate the effectiveness of steps taken to reduce the growth of molds in food and feed, methods that can accurately quantify the degree of fungal contamination of solid substrates are needed. In this study, the ergosterol assay has been evaluated by comparing the results of this assay with spore counts and hyphal length measurements made with a microscope and with CFU counts. Three fungi with different growth patterns during cultivation on a synthetic agar substrate were used in these experiments. For the nonsporulating Fusarium culmorum, there was good agreement between changes in hyphal length, CFU, and ergosterol content. Penicillium rugulosum and Rhizopus stolonifer produced many spores, and the production of spores coincided with large increases in CFU but not with increases in hyphal length or ergosterol content. Spores constituted between 3 and 5% of the total fungal mass. Changes in ergosterol level were closely related to changes in hyphal length. It was concluded that ergosterol level is a suitable marker for use in quantitatively monitoring fungal growth in solid substrates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakken L. R., Olsen R. A. Buoyant densities and dry-matter contents of microorganisms: conversion of a measured biovolume into biomass. Appl Environ Microbiol. 1983 Apr;45(4):1188–1195. doi: 10.1128/aem.45.4.1188-1195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Börjesson T., Stöllman U., Schnürer J. Volatile metabolites and other indicators of Penicillium aurantiogriseum growth on different substrates. Appl Environ Microbiol. 1990 Dec;56(12):3705–3710. doi: 10.1128/aem.56.12.3705-3710.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey F. M., MacDonald M. M., Phillips S. I. Development of monoclonal-antibody-ELISA, -DOT-BLOT and -DIP-STICK immunoassays for Humicola lanuginosa in rice. J Gen Microbiol. 1989 Feb;135(Pt 2):361–373. doi: 10.1099/00221287-135-2-361. [DOI] [PubMed] [Google Scholar]
- Jarvis B., Seiler D. A., Ould A. J., Williams A. P. Observations on the enumeration of moulds in food and feedingstuffs. J Appl Bacteriol. 1983 Oct;55(2):325–336. doi: 10.1111/j.1365-2672.1983.tb01329.x. [DOI] [PubMed] [Google Scholar]
- Müller H. M., Lehn C. Ergosterin als Mass für das Pilzwachstum in Futtermitteln. I. Mitteilung. Ergosteringehalt von Getreide. Arch Tierernahr. 1988 Mar;38(3):227–240. doi: 10.1080/17450398809428290. [DOI] [PubMed] [Google Scholar]
- Zill G., Engelhardt G., Wallnöfer P. R. Determination of ergosterol as a measure of fungal growth using Si 60 HPLC. Z Lebensm Unters Forsch. 1988 Sep;187(3):246–249. doi: 10.1007/BF01043348. [DOI] [PubMed] [Google Scholar]



