Abstract
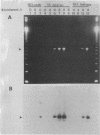
The polymerase chain reaction was used to selectively amplify sequences within the cholera toxin operon from Vibrio cholerae O1. Oysters, crabmeat, shrimp, and lettuce were seeded with V. cholerae and then homogenized or washed with alkaline peptone water, followed by short-term (6- to 8-h) enrichment. A detection limit of as few as 1 V. cholerae CFU per 10 g of food was obtained with amplification reactions from crude bacterial lysates. The method is extremely rapid and obviates the need for DNA isolation from a variety of complex food matrices.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake P. A., Wachsmuth K., Davis B. R., Bopp C. A., Chaiken B. P., Lee J. V. Toxigenic Vibrio cholerae O1 strain from Mexico identical to United States isolates. Lancet. 1983 Oct 15;2(8355):912–912. doi: 10.1016/s0140-6736(83)90894-2. [DOI] [PubMed] [Google Scholar]
- Dams E., De Wolf M., Dierick W. Nucleotide sequence analysis of the CT operon of the Vibrio cholerae classical strain 569B. Biochim Biophys Acta. 1991 Aug 27;1090(1):139–141. doi: 10.1016/0167-4781(91)90050-v. [DOI] [PubMed] [Google Scholar]
- DePaola A., Capers G. M., Motes M. L., Olsvik O., Fields P. I., Wells J., Wachsmuth I. K., Cebula T. A., Koch W. H., Khambaty F. Isolation of Latin American epidemic strain of Vibrio cholerae O1 from US Gulf Coast. Lancet. 1992 Mar 7;339(8793):624–624. doi: 10.1016/0140-6736(92)90917-r. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Popovic T., Wachsmuth K., Olsvik O. Use of polymerase chain reaction for detection of toxigenic Vibrio cholerae O1 strains from the Latin American cholera epidemic. J Clin Microbiol. 1992 Aug;30(8):2118–2121. doi: 10.1128/jcm.30.8.2118-2121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro M. L., Greenaway P. J. Nucleotide sequences within the cholera toxin operon. Nucleic Acids Res. 1983 Jun 25;11(12):3855–3861. doi: 10.1093/nar/11.12.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. E., Keasler S. P., Trucksess M. W., Feng P., Kaysner C. A., Lampel K. A. Polymerase chain reaction identification of Vibrio vulnificus in artificially contaminated oysters. Appl Environ Microbiol. 1991 Mar;57(3):707–711. doi: 10.1128/aem.57.3.707-711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Seto K., Akasaka S., Makino M. [Detection of toxigenic Vibrio cholerae O1 using polymerase chain reaction for amplifying the cholera enterotoxin gene]. Kansenshogaku Zasshi. 1990 Oct;64(10):1323–1329. doi: 10.11150/kansenshogakuzasshi1970.64.1323. [DOI] [PubMed] [Google Scholar]
- Lampel K. A., Jagow J. A., Trucksess M., Hill W. E. Polymerase chain reaction for detection of invasive Shigella flexneri in food. Appl Environ Microbiol. 1990 Jun;56(6):1536–1540. doi: 10.1128/aem.56.6.1536-1540.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman H., Kaper J. B. Nucleotide sequence analysis of the A2 and B subunits of Vibrio cholerae enterotoxin. J Biol Chem. 1983 Nov 25;258(22):13722–13726. [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Moseley S. L., Huq I., Alim A. R., So M., Samadpour-Motalebi M., Falkow S. Detection of enterotoxigenic Escherichia coli by DNA colony hybridization. J Infect Dis. 1980 Dec;142(6):892–898. doi: 10.1093/infdis/142.6.892. [DOI] [PubMed] [Google Scholar]
- Shirai H., Nishibuchi M., Ramamurthy T., Bhattacharya S. K., Pal S. C., Takeda Y. Polymerase chain reaction for detection of the cholera enterotoxin operon of Vibrio cholerae. J Clin Microbiol. 1991 Nov;29(11):2517–2521. doi: 10.1128/jcm.29.11.2517-2521.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao T., Watanabe H., Shimonishi Y. Facile identification of protein sequences by mass spectrometry. B subunit of Vibrio cholerae classical biotype Inaba 569B toxin. Eur J Biochem. 1985 Feb 1;146(3):503–508. doi: 10.1111/j.1432-1033.1985.tb08680.x. [DOI] [PubMed] [Google Scholar]
- Tauxe R. V., Blake P. A. Epidemic cholera in Latin America. JAMA. 1992 Mar 11;267(10):1388–1390. [PubMed] [Google Scholar]
- Wernars K., Delfgou E., Soentoro P. S., Notermans S. Successful approach for detection of low numbers of enterotoxigenic Escherichia coli in minced meat by using the polymerase chain reaction. Appl Environ Microbiol. 1991 Jul;57(7):1914–1919. doi: 10.1128/aem.57.7.1914-1919.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]