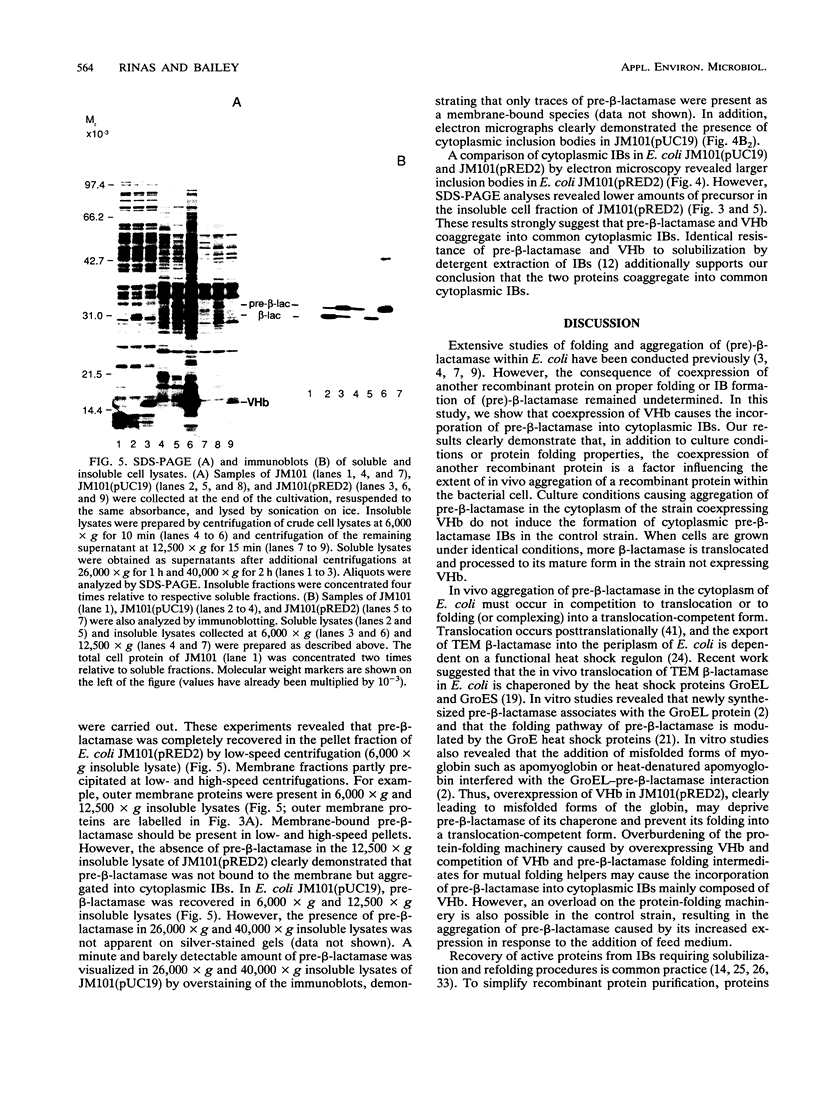
Abstract
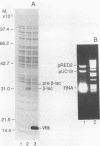
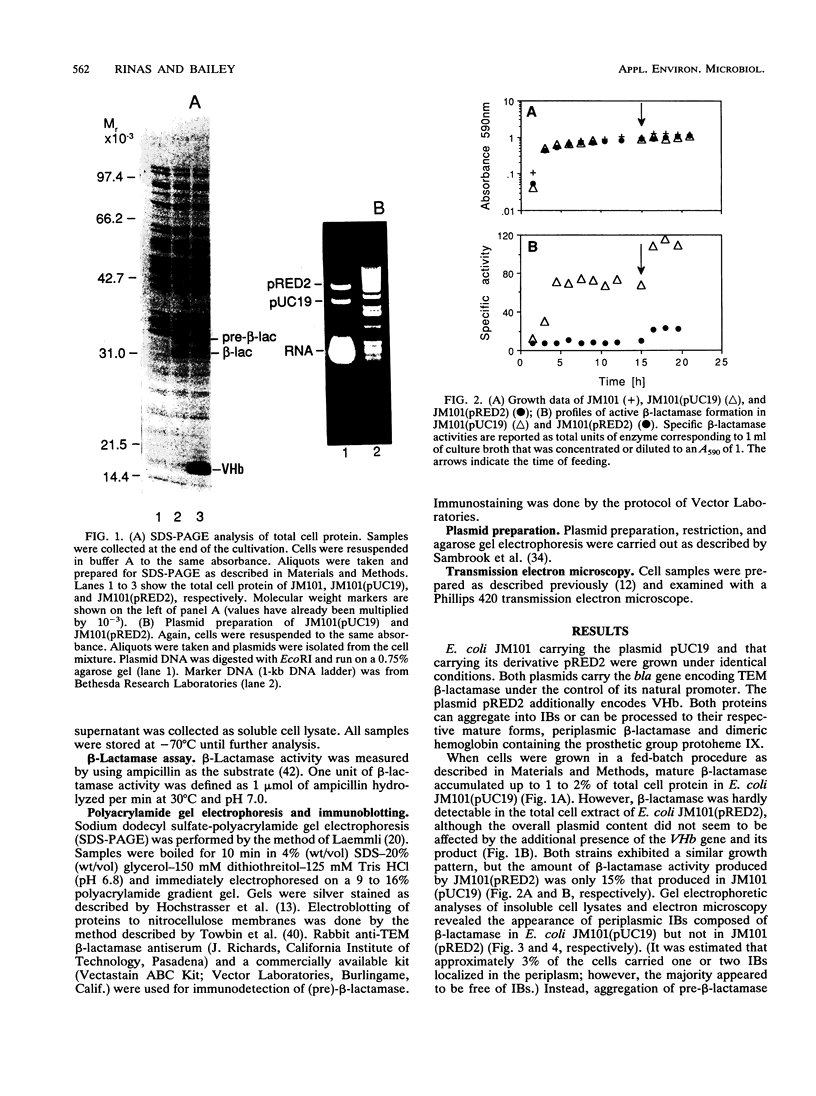
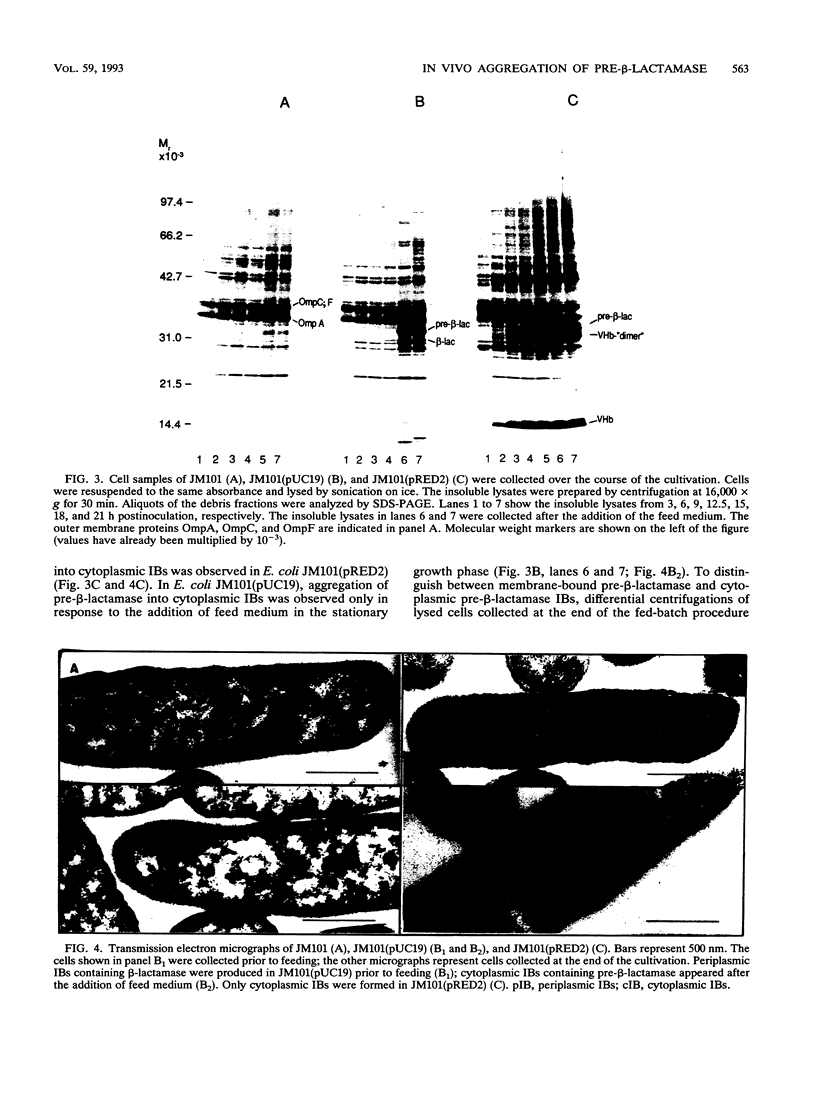
The expression of Vitreoscilla hemoglobin (VHb) in Escherichia coli JM101 (pRED2) causes the incorporation of the TEM beta-lactamase precursor into cytoplasmic inclusion bodies (IBs). Less pre-beta-lactamase is translocated and processed to its mature, periplasmic form in the strain coexpressing VHb than in the control strain E. coli JM101(pUC19) not expressing VHb. When cells are grown in a special fed-batch procedure, the formation of cytoplasmic IBs consisting of pre-beta-lactamase is also inducible in the control strain. Comparative microscopic and compositional analyses of IBs generated in E. coli JM101(pUC19) and JM101(pRED2) under identical growth conditions strongly suggest that pre-beta-lactamase and VHb coaggregate into common IBs in E. coli JM101 (pRED2).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blum P., Velligan M., Lin N., Matin A. DnaK-mediated alterations in human growth hormone protein inclusion bodies. Biotechnology (N Y) 1992 Mar;10(3):301–304. doi: 10.1038/nbt0392-301. [DOI] [PubMed] [Google Scholar]
- Bochkareva E. S., Lissin N. M., Girshovich A. S. Transient association of newly synthesized unfolded proteins with the heat-shock GroEL protein. Nature. 1988 Nov 17;336(6196):254–257. doi: 10.1038/336254a0. [DOI] [PubMed] [Google Scholar]
- Bowden G. A., Georgiou G. Folding and aggregation of beta-lactamase in the periplasmic space of Escherichia coli. J Biol Chem. 1990 Oct 5;265(28):16760–16766. [PubMed] [Google Scholar]
- Bowden G. A., Paredes A. M., Georgiou G. Structure and morphology of protein inclusion bodies in Escherichia coli. Biotechnology (N Y) 1991 Aug;9(8):725–730. doi: 10.1038/nbt0891-725. [DOI] [PubMed] [Google Scholar]
- Browner M. F., Rasor P., Tugendreich S., Fletterick R. J. Temperature-sensitive production of rabbit muscle glycogen phosphorylase in Escherichia coli. Protein Eng. 1991 Feb;4(3):351–357. doi: 10.1093/protein/4.3.351. [DOI] [PubMed] [Google Scholar]
- Carrillo N., Ceccarelli E. A., Krapp A. R., Boggio S., Ferreyra R. G., Viale A. M. Assembly of plant ferredoxin-NADP+ oxidoreductase in Escherichia coli requires GroE molecular chaperones. J Biol Chem. 1992 Aug 5;267(22):15537–15541. [PubMed] [Google Scholar]
- Chalmers J. J., Kim E., Telford J. N., Wong E. Y., Tacon W. C., Shuler M. L., Wilson D. B. Effects of temperature on Escherichia coli overproducing beta-lactamase or human epidermal growth factor. Appl Environ Microbiol. 1990 Jan;56(1):104–111. doi: 10.1128/aem.56.1.104-111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S. Increased cell buoyant densities of protein overproducing Escherichia coli cells. Biochem Biophys Res Commun. 1983 Feb 28;111(1):104–111. doi: 10.1016/s0006-291x(83)80123-5. [DOI] [PubMed] [Google Scholar]
- Georgiou G., Telford J. N., Shuler M. L., Wilson D. B. Localization of inclusion bodies in Escherichia coli overproducing beta-lactamase or alkaline phosphatase. Appl Environ Microbiol. 1986 Nov;52(5):1157–1161. doi: 10.1128/aem.52.5.1157-1161.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Gross M., Sweet R. W., Sathe G., Yokoyama S., Fasano O., Goldfarb M., Wigler M., Rosenberg M. Purification and characterization of human H-ras proteins expressed in Escherichia coli. Mol Cell Biol. 1985 May;5(5):1015–1024. doi: 10.1128/mcb.5.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart R. A., Rinas U., Bailey J. E. Protein composition of Vitreoscilla hemoglobin inclusion bodies produced in Escherichia coli. J Biol Chem. 1990 Jul 25;265(21):12728–12733. [PubMed] [Google Scholar]
- Hochstrasser D. F., Harrington M. G., Hochstrasser A. C., Miller M. J., Merril C. R. Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal Biochem. 1988 Sep;173(2):424–435. doi: 10.1016/0003-2697(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Kane J. F., Hartley D. L. Properties of recombinant protein-containing inclusion bodies in Escherichia coli. Bioprocess Technol. 1991;12:121–145. [PubMed] [Google Scholar]
- Khosla C., Bailey J. E. The Vitreoscilla hemoglobin gene: molecular cloning, nucleotide sequence and genetic expression in Escherichia coli. Mol Gen Genet. 1988 Sep;214(1):158–161. doi: 10.1007/BF00340195. [DOI] [PubMed] [Google Scholar]
- Kopetzki E., Schumacher G., Buckel P. Control of formation of active soluble or inactive insoluble baker's yeast alpha-glucosidase PI in Escherichia coli by induction and growth conditions. Mol Gen Genet. 1989 Mar;216(1):149–155. doi: 10.1007/BF00332244. [DOI] [PubMed] [Google Scholar]
- Kusukawa N., Yura T., Ueguchi C., Akiyama Y., Ito K. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 1989 Nov;8(11):3517–3521. doi: 10.1002/j.1460-2075.1989.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laminet A. A., Ziegelhoffer T., Georgopoulos C., Plückthun A. The Escherichia coli heat shock proteins GroEL and GroES modulate the folding of the beta-lactamase precursor. EMBO J. 1990 Jul;9(7):2315–2319. doi: 10.1002/j.1460-2075.1990.tb07403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Choi Y. C., Yu M. H. Effect of the N-terminal hydrophobic sequence of hepatitis B virus surface antigen on the folding and assembly of hybrid beta-galactosidase in Escherichia coli. Eur J Biochem. 1990 Jan 26;187(2):417–424. doi: 10.1111/j.1432-1033.1990.tb15320.x. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Olins P. O. Effect of overproduction of heat shock chaperones GroESL and DnaK on human procollagenase production in Escherichia coli. J Biol Chem. 1992 Feb 15;267(5):2849–2852. [PubMed] [Google Scholar]
- Marston F. A., Hartley D. L. Solubilization of protein aggregates. Methods Enzymol. 1990;182:264–276. doi: 10.1016/0076-6879(90)82022-t. [DOI] [PubMed] [Google Scholar]
- Marston F. A. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J. 1986 Nov 15;240(1):1–12. doi: 10.1042/bj2400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitraki A., Fane B., Haase-Pettingell C., Sturtevant J., King J. Global suppression of protein folding defects and inclusion body formation. Science. 1991 Jul 5;253(5015):54–58. doi: 10.1126/science.1648264. [DOI] [PubMed] [Google Scholar]
- Piatak M., Lane J. A., Laird W., Bjorn M. J., Wang A., Williams M. Expression of soluble and fully functional ricin A chain in Escherichia coli is temperature-sensitive. J Biol Chem. 1988 Apr 5;263(10):4837–4843. [PubMed] [Google Scholar]
- Rinas U., Bailey J. E. Protein compositional analysis of inclusion bodies produced in recombinant Escherichia coli. Appl Microbiol Biotechnol. 1992 Aug;37(5):609–614. doi: 10.1007/BF00240735. [DOI] [PubMed] [Google Scholar]
- Rinas U., Tsai L. B., Lyons D., Fox G. M., Stearns G., Fieschko J., Fenton D., Bailey J. E. Cysteine to serine substitutions in basic fibroblast growth factor: effect on inclusion body formation and proteolytic susceptibility during in vitro refolding. Biotechnology (N Y) 1992 Apr;10(4):435–440. doi: 10.1038/nbt0492-435. [DOI] [PubMed] [Google Scholar]
- Strandberg L., Enfors S. O. Factors influencing inclusion body formation in the production of a fused protein in Escherichia coli. Appl Environ Microbiol. 1991 Jun;57(6):1669–1674. doi: 10.1128/aem.57.6.1669-1674.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel R., Perry L. J., Veilleux C. Mutations in human interferon gamma affecting inclusion body formation identified by a general immunochemical screen. Biotechnology (N Y) 1991 Aug;9(8):731–737. doi: 10.1038/nbt0891-731. [DOI] [PubMed] [Google Scholar]
- Wynn R. M., Davie J. R., Cox R. P., Chuang D. T. Chaperonins groEL and groES promote assembly of heterotetramers (alpha 2 beta 2) of mammalian mitochondrial branched-chain alpha-keto acid decarboxylase in Escherichia coli. J Biol Chem. 1992 Jun 25;267(18):12400–12403. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- van Dijl J. M., Smith H., Bron S., Venema G. Synthesis and processing of Escherichia coli TEM-beta-lactamase and Bacillus licheniformis alpha-amylase in E. coli: the role of signal peptidase I. Mol Gen Genet. 1988 Sep;214(1):55–61. doi: 10.1007/BF00340179. [DOI] [PubMed] [Google Scholar]