Abstract
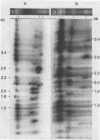
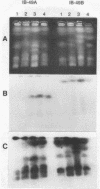
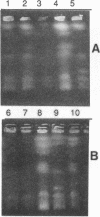
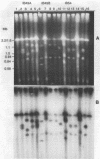
We have analyzed the karyotype of the rice blast fungus, Magnaporthe grisea, by using pulsed-filed gel electrophoresis. We tested whether the electrophoretic karyotype of an isolate was related to its pathotype, as determined by infection assays, or its genetic lineage, as determined by DNA fingerprinting. Highly reproducible electrophoretic karyotypes were obtained for a collection of U.S. and Chinese isolates representing a diverse collection of pathotypes and genetic lineages. Chromosomes ranged in size from 3 to 10 Mb. Although chromosome number was largely invariant, chromosome length polymorphisms were frequent. Minichromosomes were also found, although their presence was not ubiquitous. They ranged in number from 1 to 3 and in size from 470 kb to 2.2 Mb. Karyotypes were sufficiently variable as to obscure the obvious relatedness of isolates on the basis of pathogenicity assays or genetic lineage analysis by DNA fingerprinting. We documented that the electrophoretic karyotype of an isolate can change after prolonged serial transfer in culture and that this change did not alter the isolate's pathotype. The mechanisms bringing about karyotype variability involve deletions, translocations, and more complex rearrangements. We conclude that karyotypic variability in the rice blast fungus is a reflection of the lack of sexuality in wild populations which leads to the maintenance of neutral genomic rearrangements in clones of the fungus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Cooley R. N., Caten C. E. Variation in electrophoretic karyotype between strains of Septoria nodorum. Mol Gen Genet. 1991 Aug;228(1-2):17–23. doi: 10.1007/BF00282442. [DOI] [PubMed] [Google Scholar]
- Crawford M. S., Chumley F. G., Weaver C. G., Valent B. Characterization of the Heterokaryotic and Vegetative Diploid Phases of MAGNAPORTHE GRISEA. Genetics. 1986 Dec;114(4):1111–1129. doi: 10.1093/genetics/114.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hamer J. E., Farrall L., Orbach M. J., Valent B., Chumley F. G. Host species-specific conservation of a family of repeated DNA sequences in the genome of a fungal plant pathogen. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9981–9985. doi: 10.1073/pnas.86.24.9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer J. E. Molecular probes for rice blast disease. Science. 1991 May 3;252(5006):632–633. doi: 10.1126/science.252.5006.632. [DOI] [PubMed] [Google Scholar]
- Hamer J. E., Valent B., Chumley F. G. Mutations at the smo genetic locus affect the shape of diverse cell types in the rice blast fungus. Genetics. 1989 Jun;122(2):351–361. doi: 10.1093/genetics/122.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M., Romao J., Marchetti M. A., Hamer J. E. DNA Fingerprinting with a Dispersed Repeated Sequence Resolves Pathotype Diversity in the Rice Blast Fungus. Plant Cell. 1991 Jan;3(1):95–102. doi: 10.1105/tpc.3.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao V. P., Covert S. F., VanEtten H. D. A fungal gene for antibiotic resistance on a dispensable ("B") chromosome. Science. 1991 Dec 20;254(5039):1773–1776. doi: 10.1126/science.1763326. [DOI] [PubMed] [Google Scholar]
- Miao V. P., Matthews D. E., VanEtten H. D. Identification and chromosomal locations of a family of cytochrome P-450 genes for pisatin detoxification in the fungus Nectria haematococca. Mol Gen Genet. 1991 Apr;226(1-2):214–223. doi: 10.1007/BF00273606. [DOI] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach M. J., Vollrath D., Davis R. W., Yanofsky C. An electrophoretic karyotype of Neurospora crassa. Mol Cell Biol. 1988 Apr;8(4):1469–1473. doi: 10.1128/mcb.8.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romao J., Hamer J. E. Genetic organization of a repeated DNA sequence family in the rice blast fungus. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5316–5320. doi: 10.1073/pnas.89.12.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustchenko-Bulgac E. P., Sherman F., Hicks J. B. Chromosomal rearrangements associated with morphological mutants provide a means for genetic variation of Candida albicans. J Bacteriol. 1990 Mar;172(3):1276–1283. doi: 10.1128/jb.172.3.1276-1283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Miyamae Y., Ishida I. Variation of colony morphology and chromosomal rearrangement in Candida tropicalis pK233. J Gen Microbiol. 1991 Jan;137(1):161–167. doi: 10.1099/00221287-137-1-161. [DOI] [PubMed] [Google Scholar]
- Sweigard J. A., Chumley F. G., Valent B. Cloning and analysis of CUT1, a cutinase gene from Magnaporthe grisea. Mol Gen Genet. 1992 Mar;232(2):174–182. doi: 10.1007/BF00279994. [DOI] [PubMed] [Google Scholar]
- Talbot N. J., Oliver R. P., Coddington A. Pulsed field gel electrophoresis reveals chromosome length differences between strains of Cladosporium fulvum (syn. Fulvia fulva). Mol Gen Genet. 1991 Oct;229(2):267–272. doi: 10.1007/BF00272165. [DOI] [PubMed] [Google Scholar]
- Tzeng T. H., Lyngholm L. K., Ford C. F., Bronson C. R. A restriction fragment length polymorphism map and electrophoretic karyotype of the fungal maize pathogen Cochliobolus heterostrophus. Genetics. 1992 Jan;130(1):81–96. doi: 10.1093/genetics/130.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]