Abstract
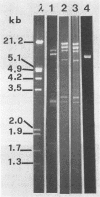
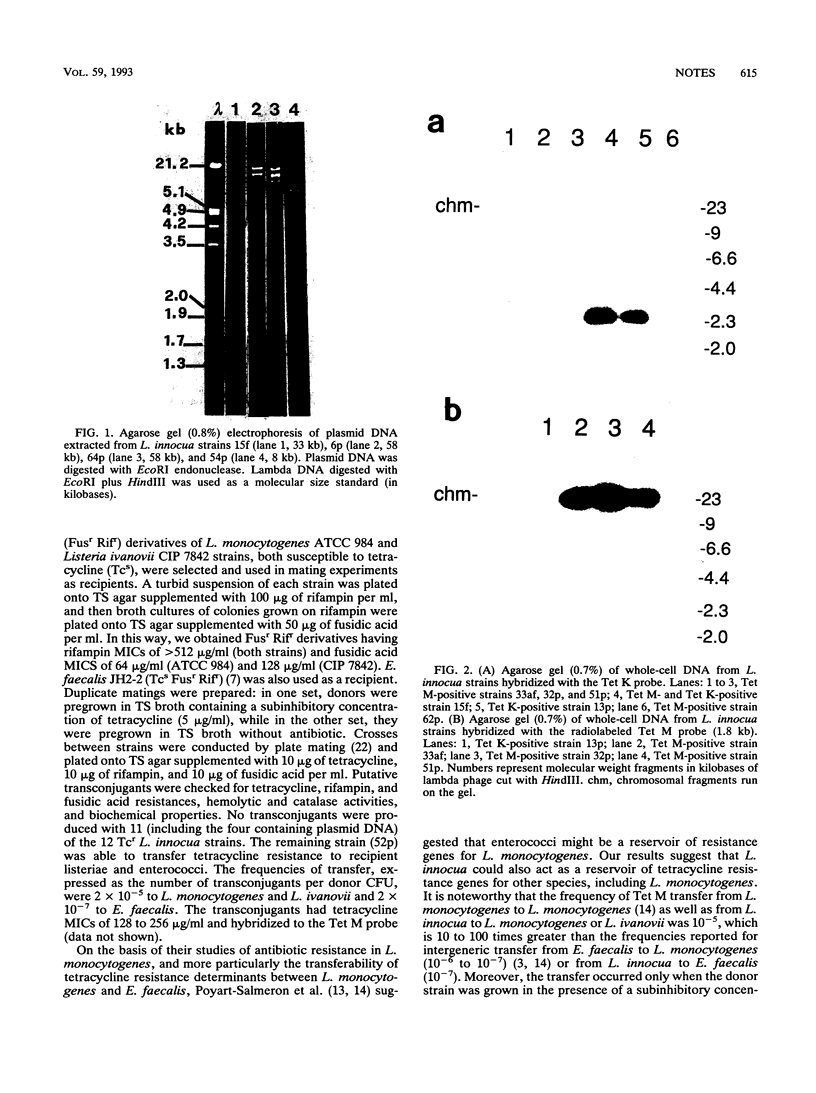
Eleven of 12 tetracycline-resistant Listeria innocua strains, isolated from chicken or turkey frankfurters and mozzarella cheese, were shown to carry DNA sequences which hybridized with the Tet M probe; of these, two strains also hybridized with Tet K. The remaining strain hybridized with the Tet K probe only. The Tet M determinant appeared to be located on the chromosome; in one case, it was transferable by conjugation to recipients Listeria monocytogenes, Listeria ivanovii, and Enterococcus faecalis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. T., Roberts M. C. Cloning and characterization of tetM gene from a Ureaplasma urealyticum strain. Antimicrob Agents Chemother. 1987 Nov;31(11):1852–1854. doi: 10.1128/aac.31.11.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet-Populaire F., Trieu-Cuot P., Dosbaa I., Andremont A., Courvalin P. Inducible transfer of conjugative transposon Tn1545 from Enterococcus faecalis to Listeria monocytogenes in the digestive tracts of gnotobiotic mice. Antimicrob Agents Chemother. 1991 Jan;35(1):185–187. doi: 10.1128/aac.35.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facinelli B., Giovanetti E., Varaldo P. E., Casolari P., Fabio U. Antibiotic resistance in foodborne Listeria. Lancet. 1991 Nov 16;338(8777):1272–1272. doi: 10.1016/0140-6736(91)92138-r. [DOI] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley D. L., Jones K. R., Tobian J. A., LeBlanc D. J., Macrina F. L. Disseminated tetracycline resistance in oral streptococci: implication of a conjugative transposon. Infect Immun. 1984 Jul;45(1):13–17. doi: 10.1128/iai.45.1.13-17.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes T. H. Some historical notes on chlortetracycline. Rev Infect Dis. 1985 Sep-Oct;7(5):702–707. doi: 10.1093/clinids/7.5.702. [DOI] [PubMed] [Google Scholar]
- MacGowen A. P., Reeves D. S., McLauchlin J. Antibiotic resistance of Listeria monocytogenes. Lancet. 1990 Aug 25;336(8713):513–514. [PubMed] [Google Scholar]
- Owen R. J., Borman P. A rapid biochemical method for purifying high molecular weight bacterial chromosomal DNA for restriction enzyme analysis. Nucleic Acids Res. 1987 Apr 24;15(8):3631–3631. doi: 10.1093/nar/15.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart-Salmeron C., Carlier C., Trieu-Cuot P., Courtieu A. L., Courvalin P. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet. 1990 Jun 16;335(8703):1422–1426. doi: 10.1016/0140-6736(90)91447-i. [DOI] [PubMed] [Google Scholar]
- Poyart-Salmeron C., Trieu-Cuot P., Carlier C., MacGowan A., McLauchlin J., Courvalin P. Genetic basis of tetracycline resistance in clinical isolates of Listeria monocytogenes. Antimicrob Agents Chemother. 1992 Feb;36(2):463–466. doi: 10.1128/aac.36.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin C., Thibaut M. C., Horovitz J., Bebear C. Multiresistant strain of Listeria monocytogenes in septic abortion. Lancet. 1990 Aug 11;336(8711):375–375. doi: 10.1016/0140-6736(90)91916-x. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., Hillier S. L. Genetic basis of tetracycline resistance in urogenital bacteria. Antimicrob Agents Chemother. 1990 Feb;34(2):261–264. doi: 10.1128/aac.34.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Kenny G. E. Conjugal transfer of transposon Tn916 from Streptococcus faecalis to Mycoplasma hominis. J Bacteriol. 1987 Aug;169(8):3836–3839. doi: 10.1128/jb.169.8.3836-3839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Brown J. T., Roberts M., Urdea M. S. The nucleotide sequence of the tetracycline resistance determinant tetM from Ureaplasma urealyticum. Nucleic Acids Res. 1988 Feb 11;16(3):1216–1217. doi: 10.1093/nar/16.3.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade P. J., Collins-Thompson D. L. Listeria, plasmids, antibiotic resistance, and food. Lancet. 1990 Oct 20;336(8721):1004–1004. doi: 10.1016/0140-6736(90)92464-s. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Hiratsuka K., Ray H., Manavathu E. K. Characterization and expression of a cloned tetracycline resistance determinant from Campylobacter jejuni plasmid pUA466. J Bacteriol. 1987 Jul;169(7):2984–2989. doi: 10.1128/jb.169.7.2984-2989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilhao R., Papadopoulou B., Courvalin P. Occurrence of the Campylobacter resistance gene tetO in Enterococcus and Streptococcus spp. Antimicrob Agents Chemother. 1988 Dec;32(12):1793–1796. doi: 10.1128/aac.32.12.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]