Abstract
Recently generated progesterone receptor (PR)-negative (PR−/−) mice provide an excellent model for dissecting the role of progesterone in the development of the mammary gland during puberty and pregnancy. However, the full extent of the mammary gland defect in these mice caused by the absence of the PR cannot be assessed, because PR−/− mice do not exhibit estrous cycles and fail to become pregnant. To circumvent this difficulty, we have transplanted PR−/− breasts into wild-type mice, and we have demonstrated that the development of the mammary gland in the absence of the PR is arrested at the stage of the simple ductal system found in the young virgin mouse. Mammary transplants lacking the PR in the stromal compartment give rise to normal alveolar growth, whereas transplants containing PR−/− epithelium conserve the abnormal phenotype. Chimeric epithelia in which PR−/− cells are in close vicinity to PR wild-type cells go through complete alveolar development to which the PR−/− cells contribute. Together, these results indicate that progesterone acts by a paracrine mechanism on a subset of mammary epithelial cells to allow for alveolar growth and that expression of the PR is not required in all the cells of the mammary epithelium in order for alveolar development to proceed normally.
The mouse provides a useful model to study mammary gland development. At the onset of puberty, a simple system of branching ducts begins growing out from the nipple area into a pad of fatty connective tissue that underlies the skin. During the luteal phase of the estrous cycles, the ductal system becomes more complex through the growth of side branches. Ductal side-branching becomes more extensive during early pregnancy, and subsequently alveolar bodies develop from these ducts, fill up the fat pad, and differentiate to become the sites of milk production.
The serum levels of the sex steroid progesterone are elevated during diestrus, the phase of luteal activity of the estrous cycle, and pregnancy. Moreover, experimental manipulation of the hormonal system has implicated this hormone as an essential stimulus required for the induction of ductal branching and for alveologenesis (1). However, the elucidation of the role of progesterone is complicated by the fact that, in the mammary epithelium, synthesis of the progesterone receptor (PR) depends on estrogen, the serum levels of which are also elevated during puberty and pregnancy. This has made it difficult to assess which developmental effects can be attributed to progesterone alone.
To dissect the role of progesterone from that played by estrogen, we generated mice lacking the PR by targeted inactivation of the PR gene in the mouse germ line (2). The mammary glands of the resulting young virgin PR−/− females show the same extent of ductal development as is seen in wild-type (wt) female mice (2). However, when wt and PR−/− virgin females were exposed to estradiol and progesterone, the wt breast tissue responded with side-branching and lobuloalveolar development, whereas the mammary glands of PR−/− females remained essentially unchanged. This suggested that PR is not required for initial ductal growth but is essential for subsequent side-branching and alveologenesis.
The administration of exogenous estrogen and progesterone, as was done in the above-described experiments and in a subsequent study extending this work (3), did not permit us to properly gauge the full spectrum of complex hormonal changes that occur during a normal pregnancy. During this period, the serum levels of a wide array of other hormones, including growth hormone, prolactin, placental lactogen, and adrenal steroids, are elevated. Moreover, the secretion of each of these hormones follows specific diurnal rhythms, and it is unlikely that injections of exogenous hormones achieve physiologic serum levels and correct local concentrations.
For these reasons, we resorted to transplanting PR−/− mammary tissues into wt animals that were subsequently impregnated. This allowed us to study the morphogenesis of the breast tissue in a hormonal environment that faithfully recapitulated that seen in pregnant, unmanipulated, wt animals. The results of previous research did not provide us with clear predictions of the outcomes of these transplantation experiments. For example, the PR is expressed in both stromal and epithelial compartments of the mammary gland (4). Within the epithelium, the distribution of the PR is variegated (5). Together, such observations provided no clear indication of the contributions of various subtypes of stromal and epithelial cells to mammary epithelial morphogenesis occurring in the presence or absence of the PR.
By grafting PR−/− epithelium or stroma in combination with PR wt stroma or epithelium, we have found that the primary target for progesterone is the mammary epithelium, while a direct response of the mammary stroma is not required in order for side-branching and lobuloalveolar development to occur. Furthermore, PR−/− mammary epithelial cells can give rise to alveoli when placed in close vicinity to PR wt epithelial cells, indicating that progesterone does not need to act directly on the alveolar cells and instead can orchestrate the morphogenetic and proliferative events of alveologenesis by affecting nearby cells in the mammary epithelium.
MATERIALS AND METHODS
Mice.
ROSA26 and RAG1−/− mice were purchased from The Jackson Laboratory. The PR mutant mice were described elsewhere (2); transcription of both A and B forms of the PR was disrupted. All mice were bred in 129SV/C57BL6 genetic background.
For PR genotyping, genomic DNA was isolated from tails and analyzed by PCR. PCR was performed by denaturing the DNA at 94°C for 1 min, followed by 30 cycles of amplification: 94°C for 1 min, 60°C for 2 min, 72°C for 1 min, and a final extension step at 72°C for 5 min. The following PR-specific primers were used: P1 (5′-TAG ACA GTG TCT TAG ACT CGT TGT TG-3′), P2 (5′-AGC AGA AAA CCG TGA ATC TTC-3′), and a neo gene-specific primer, N2 (5′-GCA TGC TCC AGA CTG CCT TGG GAA A-3′).
Presence of the β-galactosidase transgene was tested for by subjecting a piece of tail to the 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-Gal) staining procedure described below.
Whole-Breast Transplant.
Four- to 6-week-old PR+/+ or PR−/− female mice were sacrificed and their inguinal mammary glands were dissected. RAG1−/− females of the same age were anesthetized with Avertin i.p. (6). The ventral skin was incised and the abdominal muscle wall was exposed. A PR−/− and a PR+/+ mammary gland were placed onto the abdominal wall and the incision was closed with surgical staples. Three weeks after surgery the recipients were mated. They were sacrificed at parturition. The two transplanted glands and an endogenous mammary gland were analyzed by whole-mount microscopy.
Fat-Pad Transplant.
Three-week-old PR+/+, PR+/−, and PR−/− females were sacrificed and their inguinal mammary glands were exposed. The nipple-near region was removed. Into the remaining empty fat pad we injected primary mammary epithelial cells derived from ROSA26 females. The engrafted fat pads were placed onto the abdominal muscle wall of virgin RAG1−/− females.
Transplantation of Mammary Epithelium.
The fat pads of 3-week-old RAG1−/− females were cleared (see above). Pieces of mammary tissue of 1-mm diameter were removed from the nipple region of PR+/+ and PR−/− females and implanted as described before (7). Alternatively, the cleared fat pads were injected with PR+/+ and PR−/− primary cells, cultured as described in ref. 8.
Mammary Gland Whole Mounts.
The inguinal mammary glands were dissected, spread onto a glass slide, fixed in a 1:3 mixture of glacial acetic acid/100% ethanol, hydrated, stained overnight in 0.2% carmine (Sigma) and 0.5% AlK(SO4)2, dehydrated in graded solutions of ethanol, and cleared in 1:2 benzyl alcohol/benzyl benzoate (Sigma) as described previously (9).
Pictures were taken on a Leica MZ12 stereoscope with Kodak Ektachrome 160T.
X-Gal Staining.
The transplanted mammary glands were dissected, fixed for 1 hr in 4% formaldehyde in phosphate-buffered saline (PBS), washed three times over 3 hr with rinse buffer (2 mM MgCl2/0.1% sodium deoxycholate/0.2% Nonidet P-40 in PBS) and rotated in X-Gal staining solution (1 mg/ml X-Gal, 5 mM potassium ferricyanide, and 5 mM potassium ferrocyanide in rinse buffer) at 37°C for 18 hr, washed in PBS, and processed for whole-mounting as described above.
Histological Examination and Immunohistochemistry.
For histological examination of the alveolar structures the whole-mounted mammary glands were washed in 100% ethanol prior to paraffin embedment. Sections were cut at 10 μm. Anti-β-casein antiserum (10) was diluted 1:500 and applied overnight at 4°C. Biotinylated secondary antibodies were detected with a Vectastain ABC kit (Vector Laboratories).
RESULTS
Development of the Mammary Gland During Pregnancy in the Absence of the PR.
To analyze the role progesterone plays in the mammary gland during normal pregnancy, entire mammary glands from PR−/− female mice and their wt littermates were transplanted onto the abdominal muscle wall of PR wt females. The transplanted glands included both epithelial and stromal compartments. The recipient females were of the same 129SV/C57BL6 genetic background and were homozygous for the inactivated RAG1 allele (11). Females of this genotype are immunocompromised and therefore able to accept allografts. The engrafted females were mated 3 weeks after surgery and sacrificed immediately after a completed pregnancy. In all cases, the implants along with an endogenous mammary gland were analyzed by whole-mount microscopy.
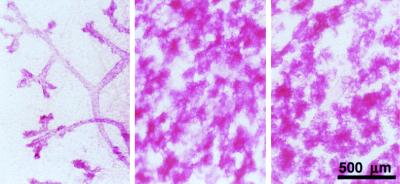
While the wt implants and endogenous glands (Fig. 1 Center and Right, respectively) showed full alveolar development at parturition, the PR−/− grafts developed only a simple ductal system (Fig. 1 Left). These observations validated the transplantation procedure. More significantly, they demonstrated, as suggested by previous reports (1, 12), that progesterone is essential for side-branching and lobuloalveolar growth and showed that, in the absence of the PR, the mammary gland fails to undergo substantial proliferation in the presence of the full array of pregnancy-associated hormones.
Figure 1.
Whole breast transplantation. Whole-mount preparations of the PR−/− (Left) and PR+/+ (Center) whole breast implant and endogenous mammary gland (Right) derived from RAG1−/− recipient mouse after parturition.
Involvement of the Stromal and the Epithelial Compartments in PR-Mediated Responses.
To address the question of whether progesterone acts on the mammary stroma or epithelium, engrafted animals were created in which either the mammary epithelium or the fat pad lacked PR because of inactivation of the PR gene. The development of the mammary gland in response to physiological hormonal stimulation was then followed.
In the mouse, the mammary epithelium grows out from the nipple into a fat pad that underlies the skin. At three weeks after birth, the epithelium of the gland has not yet penetrated extensively into the stroma and can be eliminated by removing the nipple region of the mammary gland (7). Mammary epithelial cells (MECs) that are introduced into the remaining “cleared” fat pad will give rise to a new ductal system. They can grow out from a piece of breast tissue that is placed into the fat pad (7, 13), or from single-cell suspensions that are injected into the fat pad (14).
We adapted these surgical procedures to create mammary glands that specifically lacked the PR in their stromal cells. Briefly, the nipple regions containing the mammary epithelium were removed from the fourth mammary glands of 3-week-old PR−/− females and their wt littermates. The resulting cleared fat pads were then implanted with mammary epithelium derived from a wt donor. Subsequently, the resulting reconstituted mammary glands were dissected and transplanted onto the abdominal muscle wall of RAG1−/− females.
We validated this transplantation procedure by implanting PR wt epithelium into PR wt fat pads. The resulting engrafted glands developed like the endogenous mammary glands in virgin as well as postpartum recipients, demonstrating that the engrafted fat pad had become fully vascularized when transplanted in this fashion.
The interpretation of these experiments depended upon our ability to distinguish implanted mammary epithelium from any residual endogenous epithelium that inadvertently had not been removed during the preparation of the cleared mammary fat pads. In fact, in the virgin gland, it is easy to distinguish ducts arising from implanted epithelium from those that are endogenous to this gland because of the distinctive orientations of ductal growth. Thus, the endogenous epithelium grows unidirectionally from the nipple into the fat pad, whereas the ducts arising from the implant, which we place into the center of the cleared fat pad, grow centrifugally. At parturition, however, when the fat pad is filled with alveoli, it is difficult to distinguish the two ductal systems, making it impossible to rule out that the observed epithelial structures derive from residual endogenous epithelium.
To address this difficulty, mammary epithelium derived from ROSA26 female mice was exploited (15). Mice of this transgenic strain express the β-galactosidase gene in virtually all their tissues. The mammary epithelium of these ROSA26 mice was implanted into the cleared fat pads of wt mice. When these reconstituted fat pads were subjected to an X-Gal staining procedure, the implanted ROSA26-derived epithelium turned blue and could thus be unequivocally distinguished from any endogenous epithelium, which was visualized by the red color of the carmine/alum counterstain. Together, the above-described preliminary experiments and the use of ROSA26 cells validated our transplantation procedures and our ability to study engrafted tissues without the confounding effects of residual tissue originating from the recipient breast.
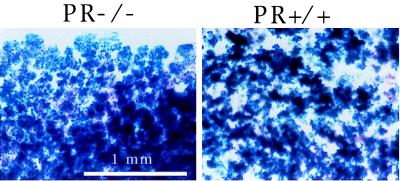
The above procedures were utilized to resolve the respective roles of stroma- and epithelium-derived PR populations in mammary gland proliferation and differentiation. First, ROSA26.PR+/+ epithelium was transplanted into cleared PR−/− fat pads; the resulting reconstituted mammary glands were then placed onto the abdominal muscle wall of a RAG1−/− recipient female. Four weeks later, the engrafted RAG1−/− recipients were mated. After they had given birth, the transplanted mammary gland and an endogenous mammary gland were analyzed by whole-mount microscopy. As can be seen in Fig. 2, the injected ROSA26-derived mammary epithelial cells grew equally well in transplanted fat pads from wt (Fig. 2 Right) and PR−/− (Fig. 2 Left) donors. This result demonstrated that the presence of the PR in the mammary stroma was not essential for the pregnancy-induced side-branching and lobuloalveolar development.
Figure 2.
Transplantation of engrafted fat pads. Whole-mount preparations of transplanted reconstituted breasts. Fat pads from PR−/− or PR+/+ mice were engrafted with ROSA26 (β-galactosidase+) PR+/+ primary mammary epithelial cells and transplanted onto the abdominal muscle wall of PR+/+.RAG1−/− recipients. the reconstituted mammary glands were removed from the recipients after parturition and stained with X-Gal before whole-mounting.
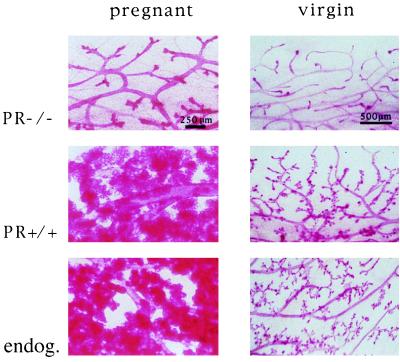
Next, we assessed the role of the PR in the epithelium independent of its function in the stroma. To do this, mammary epithelial cells derived from either PR−/− or wt donors were transplanted into the cleared mammary fat pads of wt recipients. The engrafted recipients were mated and their mammary glands were analyzed at parturition. The results of these experiments are shown in Fig. 3. Whereas the wt implant gave rise to a fully developed mammary tree, the epithelium lacking the PR grew into only a simple ductal tree (Fig. 3 Left). Similarly, when we analyzed the mammary glands of engrafted virgin females 2 months after surgery, the wt implant as well as the endogenous breasts showed side-branching, whereas the PR−/− breast had only a simple ductal system (Fig. 3 Right). Table 1 summarizes the results of these transplantation experiments. These results allowed us to conclude that the mammary epithelium is the prime target of progesterone both before and during pregnancy, and that a direct response of the mammary stroma to progesterone does not play an essential role.
Figure 3.
Transplantation of epithelium. Whole-mount preparations of mammary glands from PR+/+.RAG1−/− recipients. (Left) Preparation derived from a recipient after parturition. (Right) Preparation derived from a virgin mouse. (Top) Transplanted PR−/− epithelium. (Middle) Transplanted PR+/+ epithelium. (Bottom) Endogenous mammary gland.
Table 1.
Requirement of the PR in the stroma and/or the epithelium for alveolar development in mammary transplants analyzed post partum
| Transplant | No. samples with alveolar growth/no. successful transplants |
|---|---|
| Mammary glands in toto | |
| Stroma PR+/+/epithelium PR+/+ | 8/8 |
| Stroma PR−/−/epithelium PR−/− | 0/8 |
| Fat pad injected with PR+/+ROSA26 epithelium cells | |
| Stroma PR+/+/injected epithelium PR+/+ | 6/6 |
| Stroma PR+/−/injected epithelium PR+/+ | 8/8 |
| Stroma PR−/−/injected epithelium PR+/+ | 6/6 |
| Epithelium | |
| Stroma (host) PR+/+/epithelial transplant PR+/+ | 13/13 |
| Stroma (host) PR+/+/epithelial transplant PR−/− | 0/13 |
Role of the PR in the Development of Alveoli.
The experiments above indicated that the absence of the PR from all cells of the mammary epithelium resulted in a failure of side-branching and lobuloalveolar growth. However, they did not address the question of whether the presence of PR was required in all cells of the ductal epithelium or in only a subset of MECs in order for these morphogenetic processes to proceed normally.
To distinguish between these possibilities, we created mosaic mammary epithelia containing both PR−/− and PR+/+ MECs. The latter cells were derived from ROSA26 mice. In this case, tissue structures composed of PR+/+ cells would turn blue upon X-Gal staining when analyzed by whole-mount microscopy. Structures composed of PR−/− cells would turn red, being stained only by the carmine/alum counterstain.
Mixtures of PR+/+ and PR−/− MECs in different ratios were injected into the cleared mammary fat pads of RAG1−/− females. These mixtures were obtained either by combining single-cell suspensions derived from PR−/− and PR+/+.ROSA26 primary cultures or by mixing finely minced mammary tissues dissected from females of these two strains. Two months later, the engrafted recipients were mated, and the engrafted breasts were analyzed toward the end of pregnancy.
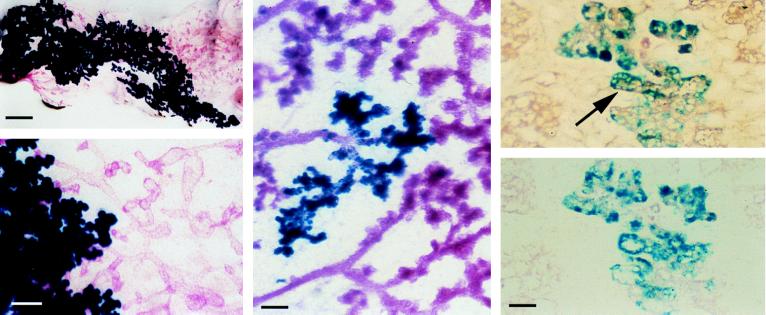
Depending on the degree of homogeneity of the injected mixture and the ratio in which the cells of the different genotypes were mixed, we found two types of chimerism. In the first type, the mammary glands showed discrete sectors having distinct phenotypes. An example, representative of 17 samples of this type of chimerism, is shown in Fig. 4. One half of the epithelial component of the mammary gland stained red while the other half stained blue; this indicated the origins of these two sectors from PR−/− and ROSA26 engrafted cells respectively. The sector composed of the PR−/− cells represents a simple ductal tree, whereas the sector composed of the PR+/+.ROSA26 cells shows extensive lobuloalveolar growth. This result demonstrated that the coexistence of MECs of PR+/+ and PR−/− in one fat pad is not sufficient to rescue the morphogenetic defect intrinsic to the PR−/− cells.
Figure 4.
Rescue of the PR−/− phenotype in PR−/− and PR+/+ chimeric epithelia. (Left) Whole-mount preparation of cleared PR+/+.RAG1−/− fat pad implanted with a mixture of PR−/− (red) epithelium and ROSA26.PR+/+ epithelium (blue) in a 1:1 ratio. The engrafted mammary gland was removed after the recipient had given birth, subjected to X-Gal staining, and whole-mounted.(Bar in Upper corresponds to 2 mm; bar in Lower, to 200 μm). (Center) Whole-mount preparation of cleared PR+/+.RAG1−/− fat pad injected with a mixture of PR−/−.ROSA26 (blue) epithelium and PR+/+ epithelium (red) injected in a 1:10 ratio, treated as for Left. (Bar corresponds to 200 μm.) (Right) Adjacent histological sections of an area with PR−/−.ROSA26 alveolar structures. (Upper) Expression of β-casein in wt and PR−/−.ROSA26 alveoli. (Lower) Control without primary antibody. Arrow indicates PR−/−.ROSA26 alveolus expressing β-casein. (Bar corresponds to 50 μm.)
Most of the chimeric epithelia that arose from single-cell suspensions in which the wt cells were in 10-fold excess over PR−/− cells showed complete lobuloalveolar development. However, at higher magnification distinct red alveoli and blue alveoli could be identified. This observation suggested but did not prove that PR−/− cells could participate in alveolar formation if they were in close proximity with wt MECs.
Any conclusions concerning the ability of the PR−/− MECs to form alveoli were clouded by the possibility that certain PR+/+.ROSA26 cells that participated in alveologenesis had failed to stain blue, thereby taking on the appearance of the PR−/− cells in the same mixed grafts. To address this issue, we crossed the β-galactosidase transgene into the PR−/− genetic background. By transplanting PR−/−.ROSA26 mammary epithelium into wt recipients and analyzing the transplanted glands after birth we were assured that the transgene did not affect the PR−/− phenotype (data not shown). Subsequently, suspensions of PR−/−.ROSA26 MECs were mixed with PR+/+ MECs lacking the β-galactosidase transgene to generate chimeric breasts. On this occasion, we looked for a result opposite to that seen previously—alveolar cells that stained blue. Indeed, as shown in Fig. 4 Center, a representative of 26 independent grafts, the mammary glands obtained from pregnant engrafted females showed areas with blue alveoli, proving conclusively that PR−/− cells can participate in the formation of alveoli if they are in close vicinity to wt epithelial cells.
To determine whether the alveolar structures constituted by PR−/− cells are functional we assessed their morphology on histological sections. As shown in Fig. 4 Right, the lumina of the blue PR−/− alveoli compare with those of wt alveoli, indicating the presence of secreted material. Similarly, secretory vacuoles are present. Immunostaining with anti-β-casein antibody revealed the expression of the milk protein (arrow, Fig. 4 Upper Right). Together these results indicate that the PR−/− alveoli are fully differentiated. Thus, the presence of the PR is required in only a portion of the MECs in order for lobuloalveolar development to occur. Moreover, these findings suggest that progesterone activates a paracrine signaling route that operates between distinct subtypes of MECs, permitting PR−/− MECs to participate directly in lobuloalveolar proliferation and differentiation.
DISCUSSION
Hormonal ablation/reconstitution experiments (1) have suggested that progesterone plays an important role in the changes that the mammary gland undergoes during early pregnancy, namely side-branching and initial alveolar growth. To determine the extent to which progesterone signaling is limiting in development, we generated mice lacking the PR gene (2). However, because the PR−/− females have multiple impairments in their reproductive functions, the specific consequences of PR inactivation on mammary gland development could not be assessed in these mice.
To circumvent this difficulty, we have used various transplantation techniques to elucidate the role of progesterone in the development of the mammary gland. In particular, we have made use of cells derived from mice carrying the β-galactosidase transgene. These cells turn blue upon X-Gal staining, making it possible to distinguish these cells histochemically from neighboring β-galactosidase-negative cells. In one experiment, this allowed us to distinguish the β-galactosidase-positive implanted MECs from the β-galactosidase-negative endogenous cells of an engrafted breast; in another setting, this procedure made it possible for us to distinguish MECs carrying two functional PR alleles from those lacking the PR.
Most transplantation experiments involving nonsyngeneic grafts have exploited nude mice as recipients. We note here in passing the utility of the RAG1−/− mice used for transplantation experiments designed to elucidate mammary gland physiology. Because nude mice have low estrogen levels, they do not represent good recipients in transplantation experiments designed specifically to gauge mammary function. In contrast, the RAG1−/− mice used here exhibit developmental defects that are strictly limited to B and T cell development (11).
Our initial experiments involving the transplantation of PR−/− mammary glands into PR+/+.RAG1−/− females were motivated by the need to assess the role of the PR in an in vivo physiologic environment in which the full array of pregnancy-associated hormonal signals was present. PR−/− mammary glands grafted into a PR+/+.RAG1−/− recipient developed only a simple ductal system, even when the host went through a series of estrous cycles and a normal pregnancy. This indicated that side-branching and lobuloalveolar growth rely on the presence of the PR, and that other signaling mechanisms operating in the breast tissue cannot compensate for the absence of the PR to allow these processes to proceed normally.
These initial results left us with two distinct scenarios. In one, both side-branching and lobuloalveolar proliferation, each in its own right, depends on the presence of progesterone. In the other, side-branching depends on progesterone, whereas lobuloalveolar growth depends on prior side-branching and is therefore only indirectly dependent on progesterone. Our analysis of a series of whole mounts of mammary glands from wt pregnant mice showed that alveoli sprouted not only from side branches (secondary ducts) but also from the primary ducts (data not shown). This finding indicated that side-branching is not an absolute prerequisite for alveolar growth. For this reason, we concluded that the PR is required for lobuloalveolar proliferation per se in addition to its demonstrated role in side-branching.
We next addressed the issue of whether progesterone needs to act on the mammary stroma, the epithelium, or both. One important clue for resolving this puzzle appeared to come from the longstanding observation that morphogenesis in many epithelial-mesenchymal organs such as the mammary gland is controlled by inductive events (16) that require cross-talk between epithelial and stromal components. In the breast in particular, the embryonic mammary mesenchyme induces the overlying epithelium to develop into the mammary bud (17). Moreover, in male embryos of various mouse strains, androgens act on the stroma to induce the involution of the mammary anlage (18, 19). The estrogen receptor is required in the mammary stroma for ductal growth to occur (20).
The role of the stroma in mediating progesterone-dependent processes in the breast has been less clear. For example, ligand-binding studies have shown that 80% of the progesterone receptors in the mouse mammary gland localize to the epithelium, while the remaining 20% are found in the stroma (4). Such observations have been compatible with models in which the epithelial cells, the stromal cells, or both cell types are required to mediate the direct responses to progesterone.
More recently, epithelial/stromal reciprocal transplantations between wt and estrogen receptor (ER)−/− and wt and PR−/− tissues have demonstrated that stromal derived ER and PR exert paracrine effects on the epithelium both in the uterus (21) and in the vagina (G. R. Cunha and B.W.O., unpublished observations). We show here that mammary glands lacking PR in the stroma undergo normal development, whereas the absence of the PR from the epithelium confers the PR−/− phenotype, indicating that the target cells of progesterone in the mammary gland are in the epithelium. While effects of progesterone on the mammary stroma cannot be excluded, they do not appear to contribute in any obvious way to the development of the ductal tree and alveoli.
Recently reported experiments in which we participated (3) yielded results that are in conflict with one aspect of the present work. These previous experiments appeared to indicate that the PR that functions within the stromal compartment exerts an effect on epithelial ductal growth, contrary to the present results, which indicate the opposite. We find the present results more compelling for several reasons. The number of transplanted animals examined here was much larger. Moreover, we have analyzed the behavior of mammary glands in a situation in which the only PR-negative tissue in engrafted animals was the mammary stroma; the earlier work, in contrast, examined the behavior of wt epithelium transplanted into the cleared PR−/− fat pad of a PR−/− host. In concordance with our conclusion, a recent immunostaining failed to detect any PR protein in the fat pad (22).
The present work together with previous observations of others (1, 12) indicates that progesterone is required for two distinct morphogenetic processes in the breast—side-branching and preparation of ductal cells for subsequent lobuloalveolar development. The precise mechanisms by which progesterone enables ductal MECs to participate in alveologenesis has been unclear. The pattern of PR expression in the mammary epithelium is inhomogeneous (5), suggesting the involvement of only a subset of ductal cells in progesterone-triggered processes. The connected issue of whether the PR-expressing cells represent the precursors of the alveolar outgrowths is addressed here.
Our observation that PR−/− cells can give rise to alveolar structures if they are in close vicinity to PR+/+ cells indicates that progesterone does not need to act directly on a ductal epithelial cell for it to participate in alveolar formation. Instead, it appears that progesterone acts on a subtype of ductal cell, causing it to release paracrine signals that permit other nearby epithelial cells to participate directly in lobuloalveolar proliferation.
The present work provides no indication about the nature of the paracrine signal released by the progesterone-activated ductal cell. However, the observation that close apposition of PR-positive with PR-negative cells is required to rescue the PR−/− phenotype indicates that the signal, whatever its biochemical nature, is transmitted only over short intercellular distances. Factors that are tightly associated with the extracellular matrix such as wnt proteins and fibroblast growth factors, which are differentially expressed during mammary gland development (23, 24), are attractive candidates for conveying such paracrine signals.
Our data provide no indication whether or not these paracrine signals communicate directly between the progesterone-activated ductal cells and closely apposed alveolar precursor cells. It remains equally possible that the progesterone-activated ductal cell communicates with the stroma; the latter, in turn, may pass on a signal directly to the alveolar precursor cells as suggested by others (25). The use of tissue reconstitution techniques and genetically altered cells should allow the further dissection of the molecular mechanisms of mammary morphogenesis over the next several years.
Acknowledgments
We thank Ms. Frances Kittrell and Dr. Daniel Medina for continued advice, Ms. Gouqingge for technical assistance, Dr. Ernst Reichmann for the generous gift of β-casein antiserum and the Dr. Mildred-Scheel foundation for their support. This work was supported by grants from the Department of the Army, Breast Cancer Research Program, the G. Harold & Leila Y. Mathers Charitable Foundation, and National Cancer Institute Grant OIG R35CA39826.
ABBREVIATIONS
- PR
progesterone receptor
- wt
wild-type
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactopyranoside
- MEC
mammary epithelial cell
References
- 1.Nandi S. J Natl Cancer Inst. 1958;21:1039–1063. [PubMed] [Google Scholar]
- 2.Lydon J P, De Mayo F J, Funk C R, Mani S K, Hughes A R, Montgomery C A, Jr, Shyamala G, Conneely O M, O’Malley B W. Genes & Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys R, R, Lydon J, O’Malley B W, Rosen J M. Mol Endocrinol. 1997;11:801–811. doi: 10.1210/mend.11.6.9891. [DOI] [PubMed] [Google Scholar]
- 4.Haslam S Z, Shyamala G. Endocrinology. 1981;108:825–830. doi: 10.1210/endo-108-3-825. [DOI] [PubMed] [Google Scholar]
- 5.Silberstein G B, Van Horn K, Shyamala G, Daniel C W. Cell Growth Differ. 1996;7:945–952. [PubMed] [Google Scholar]
- 6.Hogan B L M, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. 2nd Ed. Plainview, New York: Cold Spring Harbor Lab. Press; 1995. [Google Scholar]
- 7.DeOme, K. B., Faulkin, L. J., Jr., Bern, H. A. & Blair, P. B. (1959) Cancer Res. 511–520. [PubMed]
- 8.Kittrell F S, Oborn C J, Medina D. Cancer Res. 1992;52:1924–1932. [PubMed] [Google Scholar]
- 9.Wang S, Counterman L J, Haslam S Z. Endocrinology. 1990;127:2183–2189. doi: 10.1210/endo-127-5-2183. [DOI] [PubMed] [Google Scholar]
- 10.Reichmann E, Groner B, Friis R R. J Cell Biol. 1989;108:1127–1138. doi: 10.1083/jcb.108.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 12.Lyons W R. Proc R Soc London Ser B. 1958;149:303–325. doi: 10.1098/rspb.1958.0071. [DOI] [PubMed] [Google Scholar]
- 13.Daniel, C. W., Shannon, J. M. & Cunha, G. R. (198) Mech. Ageing Dev. 23, 259–264. [DOI] [PubMed]
- 14.Daniel C W, DeOme Science. 1965;149:634–636. doi: 10.1126/science.149.3684.634. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich G, Soriano P. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- 16.Grobstein C. J Exp Zool. 1955;130:319–340. [Google Scholar]
- 17.Propper A. Ann Embryol Morphol. 1968;2:151–160. [Google Scholar]
- 18.Kratochwil K, Schwartz P. Proc Natl Acad Sci USA. 1976;73:4041–4044. doi: 10.1073/pnas.73.11.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drews U, Drews U. Cell. 1977;10:401–404. doi: 10.1016/0092-8674(77)90027-7. [DOI] [PubMed] [Google Scholar]
- 20.Cunha G R, Young P, Hom Y K, Cooke P S, Taylor J A, Lubahn D B. J Mammary Gland Biol Neoplasia. 1997;2:393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- 21.Cooke P S, Buchanan D, L, Young P, Setiawan T, Brody J, Korach K S, Taylor J, Lubahn D B, Cunha G R. Proc Natl Acad Sci USA. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shyamala G, Barcellos-Hoff M H, Toft D, Yang X. J Steroid Biochem Mol Biol. 1997;63:251–259. doi: 10.1016/s0960-0760(97)00128-3. [DOI] [PubMed] [Google Scholar]
- 23.Gavin B J, McMahon A P. Mol Cell Biol. 1992;12:2418–2423. doi: 10.1128/mcb.12.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman-Krnacik S, Rosen J M. Mol Endocrinol. 1994;8:218–229. doi: 10.1210/mend.8.2.8170478. [DOI] [PubMed] [Google Scholar]
- 25.Birchmeier C, Sonnenberg E, Birchmeier W. BioEssays. 1993;15:185–190. doi: 10.1002/bies.950150307. [DOI] [PubMed] [Google Scholar]