Abstract
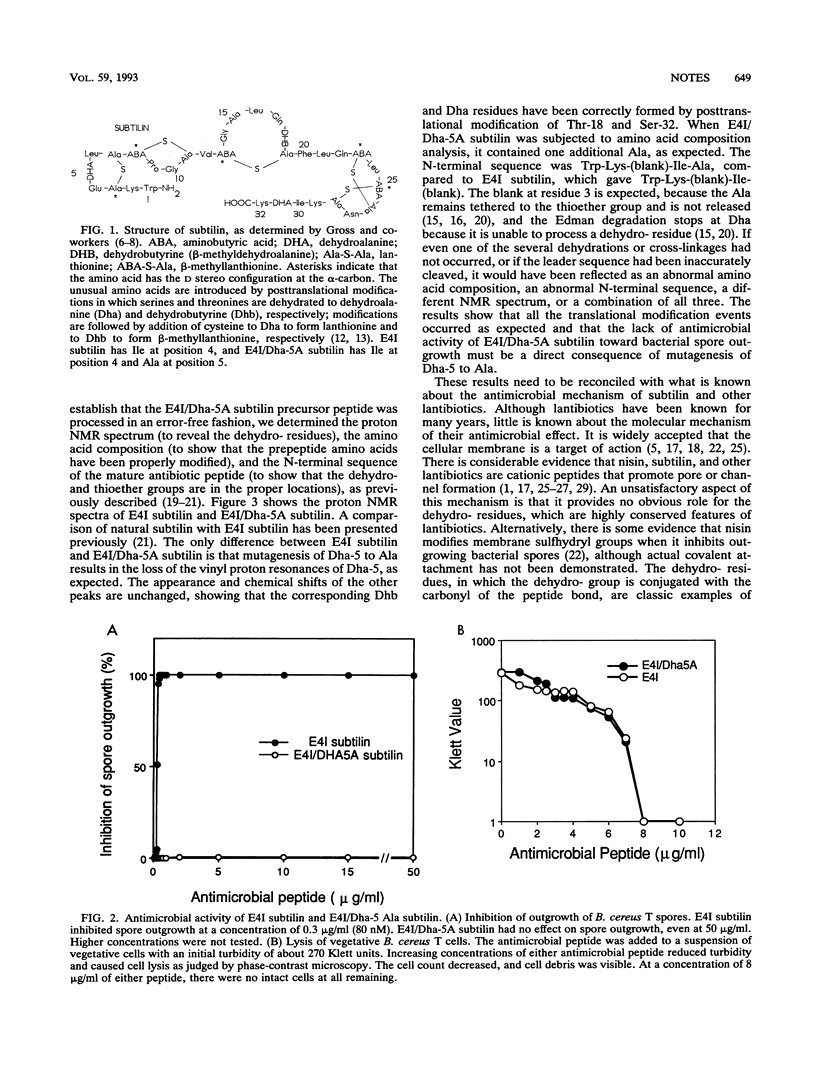
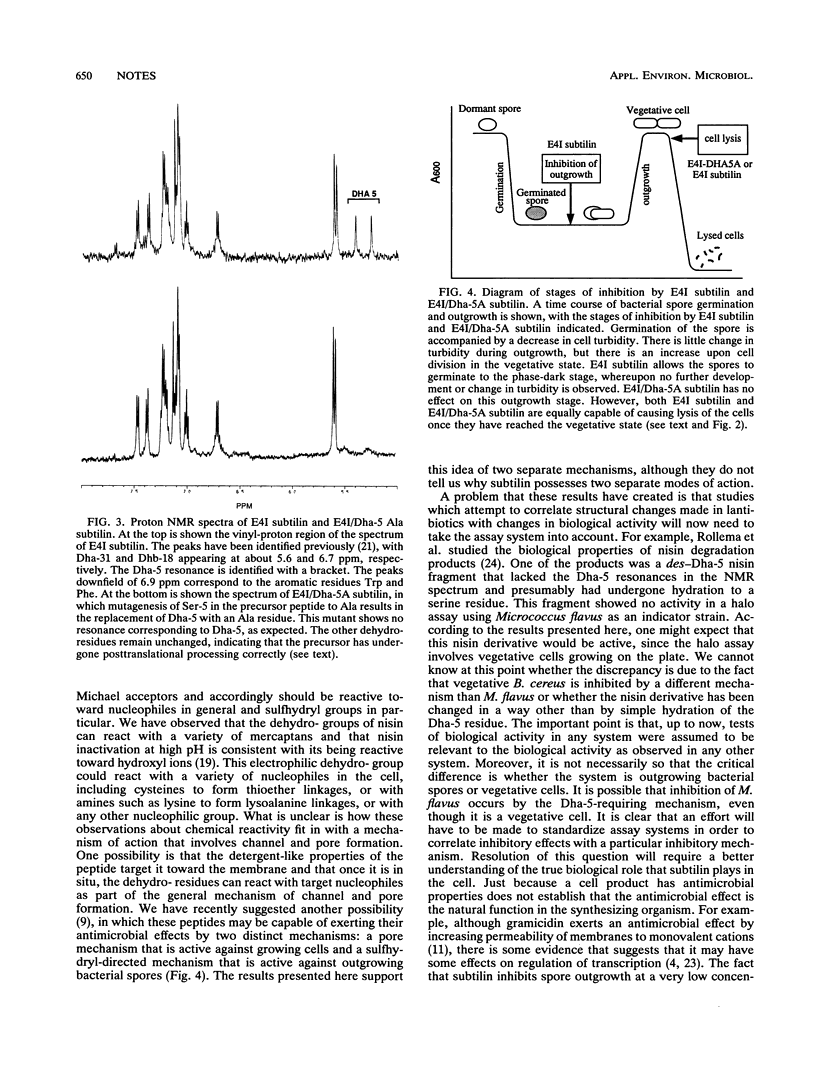
Subtilin is a ribosomally synthesized antimicrobial peptide that contains several unusual amino acids as a result of posttranslational modifications. Site-directed mutagenesis was employed to construct a structural variant of subtilin in which the unusual dehydroalanine (Dha) residue at position 5 was changed to alanine. Proton nuclear magnetic resonance spectroscopy, amino acid composition, and N-terminal sequence analysis established that the mutation did not disrupt posttranslational processing of the precursor peptide. This mutant subtilin was devoid of antimicrobial activity as assessed by its lack of inhibitory effects on outgrowth of Bacillus cereus T spores. However, this same mutant subtilin was fully active with respect to its ability to induce lysis of vegetative B. cereus T cells. Because an intact Dha-5 residue is required in the one instance but not in the other, it was concluded that the molecular mechanism by which subtilin inhibits (without lysis) spore outgrowth is not the same as the mechanism by which it inhibits (with lysis) vegetative cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bierbaum G., Sahl H. G. Influence of cationic peptides on the activity of the autolytic endo-beta-N-acetylglucosaminidase of Staphylococcus simulans 22. FEMS Microbiol Lett. 1989 Apr;49(2-3):223–227. doi: 10.1016/0378-1097(89)90042-6. [DOI] [PubMed] [Google Scholar]
- Fisher R., Blumenthal T. An interaction between gramicidin and the sigma subunit of RNA polymerase. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1045–1048. doi: 10.1073/pnas.79.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F. H., Abee T., Konings W. N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991 Aug;57(8):2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E., Kiltz H. H., Nebelin E. Subtilin, VI. Die Struktur des Subtilins. Hoppe Seylers Z Physiol Chem. 1973 Jul;354(7):810–812. [PubMed] [Google Scholar]
- Hansen J. N., Levin R. A. Effect of some inhibitors derived from nitrite on marcomolecular synthesis in Bacillus cereus. Appl Microbiol. 1975 Nov;30(5):862–869. doi: 10.1128/am.30.5.862-869.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Gramicidin, valinomycin, and cation permeability of Streptococcus faecalis. J Bacteriol. 1967 Jul;94(1):53–60. doi: 10.1128/jb.94.1.53-60.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. C. Synthesis of the antibiotic nisin: formation of lanthionine and beta-methyl-lanthionine. Biochim Biophys Acta. 1969 Jun 17;184(1):216–219. doi: 10.1016/0304-4165(69)90121-4. [DOI] [PubMed] [Google Scholar]
- Ingram L. A ribosomal mechanism for synthesis of peptides related to nisin. Biochim Biophys Acta. 1970 Nov 12;224(1):263–265. doi: 10.1016/0005-2787(70)90642-8. [DOI] [PubMed] [Google Scholar]
- Kellner R., Jung G., Hörner T., Zähner H., Schnell N., Entian K. D., Götz F. Gallidermin: a new lanthionine-containing polypeptide antibiotic. Eur J Biochem. 1988 Oct 15;177(1):53–59. doi: 10.1111/j.1432-1033.1988.tb14344.x. [DOI] [PubMed] [Google Scholar]
- Kordel M., Benz R., Sahl H. G. Mode of action of the staphylococcinlike peptide Pep 5: voltage-dependent depolarization of bacterial and artificial membranes. J Bacteriol. 1988 Jan;170(1):84–88. doi: 10.1128/jb.170.1.84-88.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordel M., Schüller F., Sahl H. G. Interaction of the pore forming-peptide antibiotics Pep 5, nisin and subtilin with non-energized liposomes. FEBS Lett. 1989 Feb 13;244(1):99–102. doi: 10.1016/0014-5793(89)81171-8. [DOI] [PubMed] [Google Scholar]
- Liu W., Hansen J. N. Conversion of Bacillus subtilis 168 to a subtilin producer by competence transformation. J Bacteriol. 1991 Nov;173(22):7387–7390. doi: 10.1128/jb.173.22.7387-7390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Hansen J. N. Enhancement of the chemical and antimicrobial properties of subtilin by site-directed mutagenesis. J Biol Chem. 1992 Dec 15;267(35):25078–25085. [PubMed] [Google Scholar]
- Liu W., Hansen J. N. Some chemical and physical properties of nisin, a small-protein antibiotic produced by Lactococcus lactis. Appl Environ Microbiol. 1990 Aug;56(8):2551–2558. doi: 10.1128/aem.56.8.2551-2558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. L., Walsh R. C., Hansen J. N. Identification and characterization of some bacterial membrane sulfhydryl groups which are targets of bacteriostatic and antibiotic action. J Biol Chem. 1984 Nov 10;259(21):13590–13594. [PubMed] [Google Scholar]
- Ristow H., Schazschneider B., Bauer K., Kleikauf H. Tyrocidine and the linear gramicidin. Do these peptide antibiotics play an antagonistic regulative role in sporulation? Biochim Biophys Acta. 1975 May 1;390(2):246–252. [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G. Bactericidal cationic peptides involved in bacterial antagonism and host defence. Microbiol Sci. 1985 Jul;2(7):212–217. [PubMed] [Google Scholar]
- Sahl H. G. Influence of the staphylococcinlike peptide Pep 5 on membrane potential of bacterial cells and cytoplasmic membrane vesicles. J Bacteriol. 1985 May;162(2):833–836. doi: 10.1128/jb.162.2.833-836.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell N., Entian K. D., Schneider U., Götz F., Zähner H., Kellner R., Jung G. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature. 1988 May 19;333(6170):276–278. doi: 10.1038/333276a0. [DOI] [PubMed] [Google Scholar]
- Schüller F., Benz R., Sahl H. G. The peptide antibiotic subtilin acts by formation of voltage-dependent multi-state pores in bacterial and artificial membranes. Eur J Biochem. 1989 Jun 1;182(1):181–186. doi: 10.1111/j.1432-1033.1989.tb14815.x. [DOI] [PubMed] [Google Scholar]
- VARY J. C., HALVORSON H. O. KINETICS OF GERMINATION OF BACILLUS SPORES. J Bacteriol. 1965 May;89:1340–1347. doi: 10.1128/jb.89.5.1340-1347.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


