Abstract
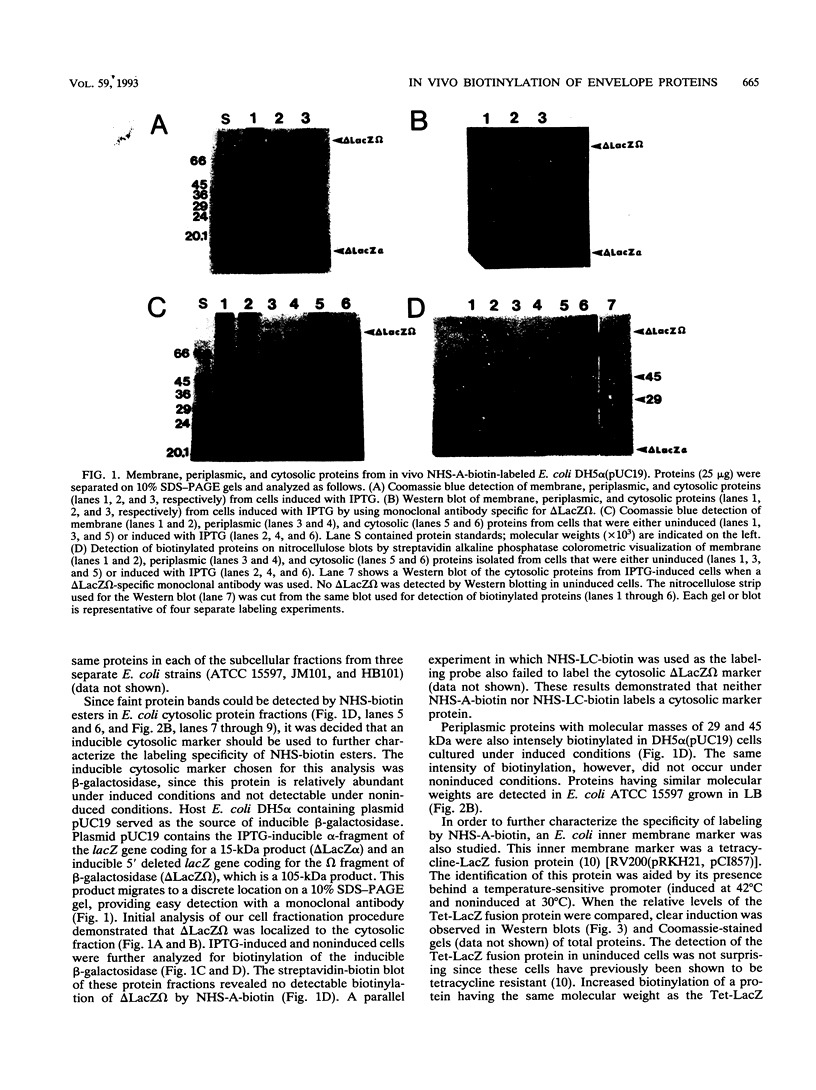
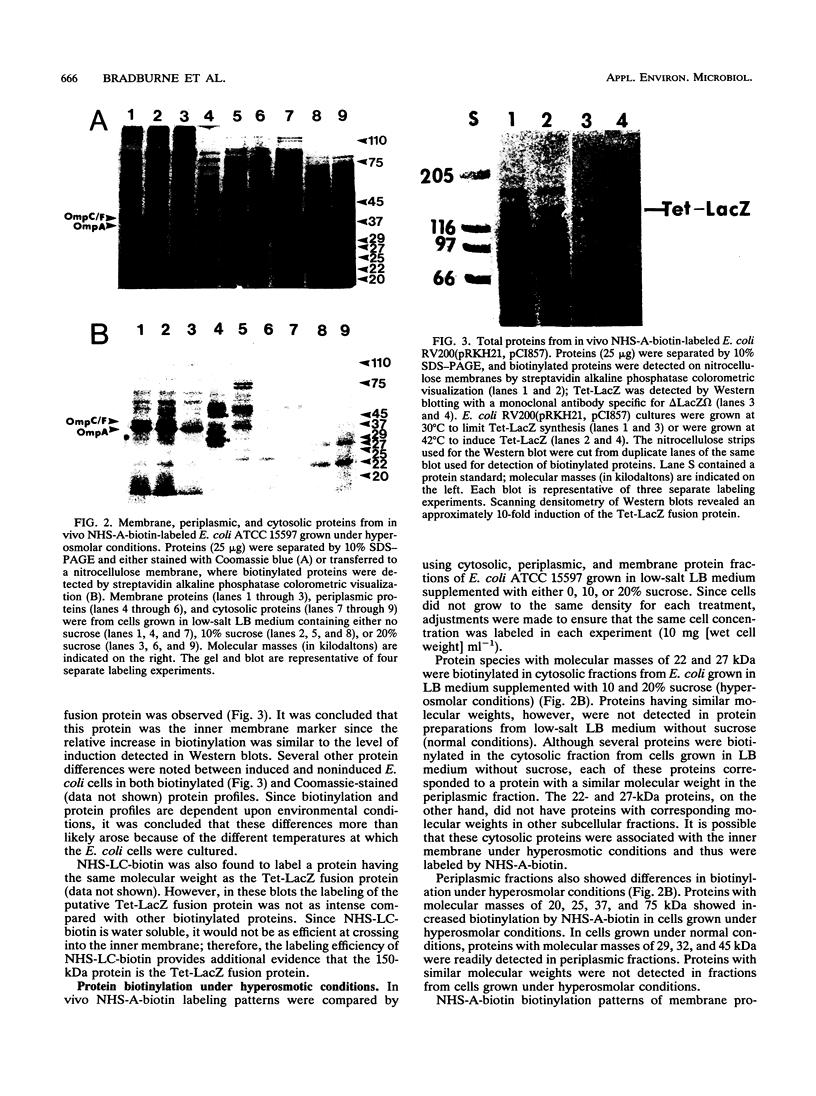
The primary amine coupling reagents succinimidyl-6-biotinamido-hexanoate (NHS-A-biotin) and sulfosuccinimidyl-6-biotinamido-hexanoate (NHS-LC-biotin) were tested for their ability to selectively label Escherichia coli cell envelope proteins in vivo. Probe localization was determined by examining membrane, periplasmic, and cytosolic protein fractions. Both hydrophobic NHS-A-biotin and hydrophilic NHS-LC-biotin were shown to preferentially label outer membrane, periplasmic, and inner membrane proteins. NHS-A- and NHS-LC-biotin were also shown to label a specific inner membrane marker protein (Tet-LacZ). Both probes, however, failed to label a cytosolic marker (the omega fragment of beta-galactosidase). The labeling procedure was also used to label E. coli cells grown in low-salt Luria broth medium supplemented with 0, 10, and 20% sucrose. Outer membrane protein A (OmpA) and OmpC were labeled by both NHS-A- and NHS-LC-biotin at all three sucrose concentrations. In contrast, OmpF was labeled by NHS-A-biotin but not by NHS-LC-biotin in media containing 0 and 10% sucrose. OmpF was not labeled by either NHS-A- or NHS-LC-biotin in E. coli cells grown in medium containing 20% sucrose. Coomassie-stained gels, however, revealed similar quantities of OmpF in E. coli cells grown at all three sucrose concentrations. These data indicate that there was a change in outer membrane structure due to increased osmolarity, which limits accessibility of NHS-A-biotin to OmpF.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Prody C., Kustu S. Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J Bacteriol. 1984 Dec;160(3):1181–1183. doi: 10.1128/jb.160.3.1181-1183.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron A., May G., Bremer E., Villarejo M. Regulation of envelope protein composition during adaptation to osmotic stress in Escherichia coli. J Bacteriol. 1986 Aug;167(2):433–438. doi: 10.1128/jb.167.2.433-438.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Ben-Hur H., Wilchek M. Enzyme-based detection of glycoproteins on blot transfers using avidin-biotin technology. Anal Biochem. 1987 Feb 15;161(1):123–131. doi: 10.1016/0003-2697(87)90661-0. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- Bayer E. A., Zalis M. G., Wilchek M. 3-(N-Maleimido-propionyl)biocytin: a versatile thiol-specific biotinylating reagent. Anal Biochem. 1985 Sep;149(2):529–536. doi: 10.1016/0003-2697(85)90609-8. [DOI] [PubMed] [Google Scholar]
- Busch G., Hoder D., Reutter W., Tauber R. Selective isolation of individual cell surface proteins from tissue culture cells by a cleavable biotin label. Eur J Cell Biol. 1989 Dec;50(2):257–262. [PubMed] [Google Scholar]
- Graeme-Cook K. A. The regulation of porin expression in Escherichia coli: effect of turgor stress. FEMS Microbiol Lett. 1991 Apr 15;63(2-3):219–223. doi: 10.1016/0378-1097(91)90089-s. [DOI] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Grimes H. D., Slay R. M., Hodges T. K. Plant Plasma Membrane Proteins : II. Biotinylation of Daucus Carota Protoplasts and Detection of Plasma Membrane Polypeptides after Sds-Page. Plant Physiol. 1988 Oct;88(2):444–449. doi: 10.1104/pp.88.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman R. K., McMurry L. M., Levy S. B. Overproduction and purification of the Tn10-specified inner membrane tetracycline resistance protein Tet using fusions to beta-galactosidase. Mol Microbiol. 1990 Aug;4(8):1241–1251. doi: 10.1111/j.1365-2958.1990.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Finn F. M., Friesen H. J., Diaconescu C., Zahn H. Biotinylinsulins as potential tools for receptor studies. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2697–2700. doi: 10.1073/pnas.74.7.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalls H. M., Goodloe-Holland C. M., Luna E. J. Junctional plasma membrane domains isolated from aggregating Dictyostelium discoideum amebae. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4779–4783. doi: 10.1073/pnas.83.13.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Orr G. A. The use of the 2-iminobiotin-avidin interaction for the selective retrieval of labeled plasma membrane components. J Biol Chem. 1981 Jan 25;256(2):761–766. [PubMed] [Google Scholar]
- Staros J. V. N-hydroxysulfosuccinimide active esters: bis(N-hydroxysulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant, protein cross-linkers. Biochemistry. 1982 Aug 17;21(17):3950–3955. doi: 10.1021/bi00260a008. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]