Abstract
An extracellular protein complex was isolated from the supernatant of a pectin-limited continuous culture of Clostridium thermosaccharolyticum Haren. The complex possessed both pectin methylesterase (EC 3.1.1.11) and exo-poly-alpha-galacturonate hydrolase (EC 3.2.1.82) activity and produced digalacturonate from the nonreducing end of the pectin chain. The protein consisted of 230- and 25-kDa subunits. The large subunit contained 10% (wt/wt) sugars (N-acetylgalactosamine and galactose). Under physiological conditions both activities acted in a coordinated manner: the ratio between methanol and digalacturonate released during degradation was constant and equal to the degree of esterification of the pectin used. Prolonged incubation of the enzyme with pectin led to a nondialyzable fraction that was enriched in neutral sugars, such as arabinose, rhamnose, and galactose; the high rhamnose/galacturonic acid ratio was indicative of hairy region-like structures. The smallest substrate utilized by the hydrolase was a tetragalacturonate. Vmax with oligogalacturonates increased with increasing chain length. The Km and Vmax for the polygalacturonate hydrolase with citrus pectate as a substrate were 0.8 g liter-1 and 180 mumol min-1 mg of protein-1, respectively. The Km and Vmax for the esterase with citrus pectin as a substrate were 1.2 g liter-1 and 440 mumol min-1 mg of protein-1, respectively. The temperature optima for the hydrolase and esterase were 70 and 60 degrees C, respectively. Both enzyme activities were stable for more than 1 h at 70 degrees C. The exo-polygalacturonate hydrolase of Clostridium thermosulfurogenes was partially purified while the methylesterase was also copurified.
Full text
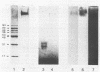
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Collmer A., Whalen C. H., Beer S. V., Bateman D. F. An exo-poly-alpha-D-galacturonosidase implicated in the regulation of extracellular pectate lyase production in Erwinia chrysanthemi. J Bacteriol. 1982 Feb;149(2):626–634. doi: 10.1128/jb.149.2.626-634.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank R. H., Wade G. C. Detection of pectic enzymes in pectin-acrylamide gels. Anal Biochem. 1980 Sep 1;107(1):177–181. doi: 10.1016/0003-2697(80)90508-4. [DOI] [PubMed] [Google Scholar]
- Dellapenna D., Bennett A. B. In vitro synthesis and processing of tomato fruit polygalacturonase. Plant Physiol. 1988 Apr;86(4):1057–1063. doi: 10.1104/pp.86.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dygert S., Li L. H., Florida D., Thoma J. A. Determination of reducing sugar with improved precision. Anal Biochem. 1965 Dec;13(3):367–374. doi: 10.1016/0003-2697(65)90327-1. [DOI] [PubMed] [Google Scholar]
- Gerard C. Purification of glycoproteins. Methods Enzymol. 1990;182:529–539. doi: 10.1016/0076-6879(90)82042-z. [DOI] [PubMed] [Google Scholar]
- Gerwig G. J., de Waard P., Kamerling J. P., Vliegenthart J. F., Morgenstern E., Lamed R., Bayer E. A. Novel O-linked carbohydrate chains in the cellulase complex (cellulosome) of Clostridium thermocellum. 3-O-Methyl-N-acetylglucosamine as a constituent of a glycoprotein. J Biol Chem. 1989 Jan 15;264(2):1027–1035. [PubMed] [Google Scholar]
- Heijthuijsen J. H., Hansen T. A. Betaine fermentation and oxidation by marine desulfuromonas strains. Appl Environ Microbiol. 1989 Apr;55(4):965–969. doi: 10.1128/aem.55.4.965-969.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichova K., Wojciechowicz M., Ziołecki A. The pectinolytic enzyme of Selenomonas ruminantium. J Appl Bacteriol. 1989 Feb;66(2):169–174. doi: 10.1111/j.1365-2672.1989.tb02466.x. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- KOLLER A., NEUKOM H. DETECTION OF OLIGOGALACTURONIC ACIDS BY THIN-LAYER CHROMATOGRAPHY. Biochim Biophys Acta. 1964 Nov 1;83:366–367. doi: 10.1016/0926-6526(64)90020-5. [DOI] [PubMed] [Google Scholar]
- Karbassi A., Vaughn R. H. Purification and properties of polygalacturonic acid trans-eliminase from Bacillus stearothermophilus. Can J Microbiol. 1980 Mar;26(3):377–384. doi: 10.1139/m80-061. [DOI] [PubMed] [Google Scholar]
- Kester H. C., Visser J. Purification and characterization of polygalacturonases produced by the hyphal fungus Aspergillus niger. Biotechnol Appl Biochem. 1990 Apr;12(2):150–160. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M., Miller L., Macmillan J. D. Similarities in the action patterns of exopolygalacturonate lyase and pectinesterase from Clostridium multifermentans. J Bacteriol. 1970 Sep;103(3):595–600. doi: 10.1128/jb.103.3.595-600.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melasniemi H. Purification and some properties of the extracellular alpha-amylase-pullulanase produced by Clostridium thermohydrosulfuricum. Biochem J. 1988 Mar 15;250(3):813–818. doi: 10.1042/bj2500813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner P., Christian R., Kolbe J., Schulz G., Sleytr U. B. Analysis of a novel linkage unit of O-linked carbohydrates from the crystalline surface layer glycoprotein of Clostridium thermohydrosulfuricum S102-70. J Bacteriol. 1992 Apr;174(7):2236–2240. doi: 10.1128/jb.174.7.2236-2240.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner P., Sleytr U. B. Bacterial surface layer glycoproteins. Glycobiology. 1991 Dec;1(6):545–551. doi: 10.1093/glycob/1.6.545. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Rexová-Benková L., Markovic O. Pectic enzymes. Adv Carbohydr Chem Biochem. 1976;33:323–385. doi: 10.1016/s0065-2318(08)60285-1. [DOI] [PubMed] [Google Scholar]
- Ried J. L., Collmer A. Activity stain for rapid characterization of pectic enzymes in isoelectric focusing and sodium dodecyl sulfate-polyacrylamide gels. Appl Environ Microbiol. 1985 Sep;50(3):615–622. doi: 10.1128/aem.50.3.615-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- SOMOGYI L. P., ROMANI R. J. A SIMPLIFIED TECHNIQUE FOR THE DETERMINATION OF PECTIN METHYLESTERASE ACTIVITY. Anal Biochem. 1964 Apr;7:498–501. doi: 10.1016/0003-2697(64)90159-9. [DOI] [PubMed] [Google Scholar]
- Sheiman M. I., Macmillan J. D., Miller L., Chase T., Jr Coordinated action of pectinesterase and polygalacturonate lyase complex of Clostridium multifermentans. Eur J Biochem. 1976 May 1;64(2):565–572. doi: 10.1111/j.1432-1033.1976.tb10336.x. [DOI] [PubMed] [Google Scholar]
- Spök A., Stubenrauch G., Schörgendorfer K., Schwab H. Molecular cloning and sequencing of a pectinesterase gene from Pseudomonas solanacearum. J Gen Microbiol. 1991 Jan;137(1):131–140. doi: 10.1099/00221287-137-1-131. [DOI] [PubMed] [Google Scholar]