Abstract
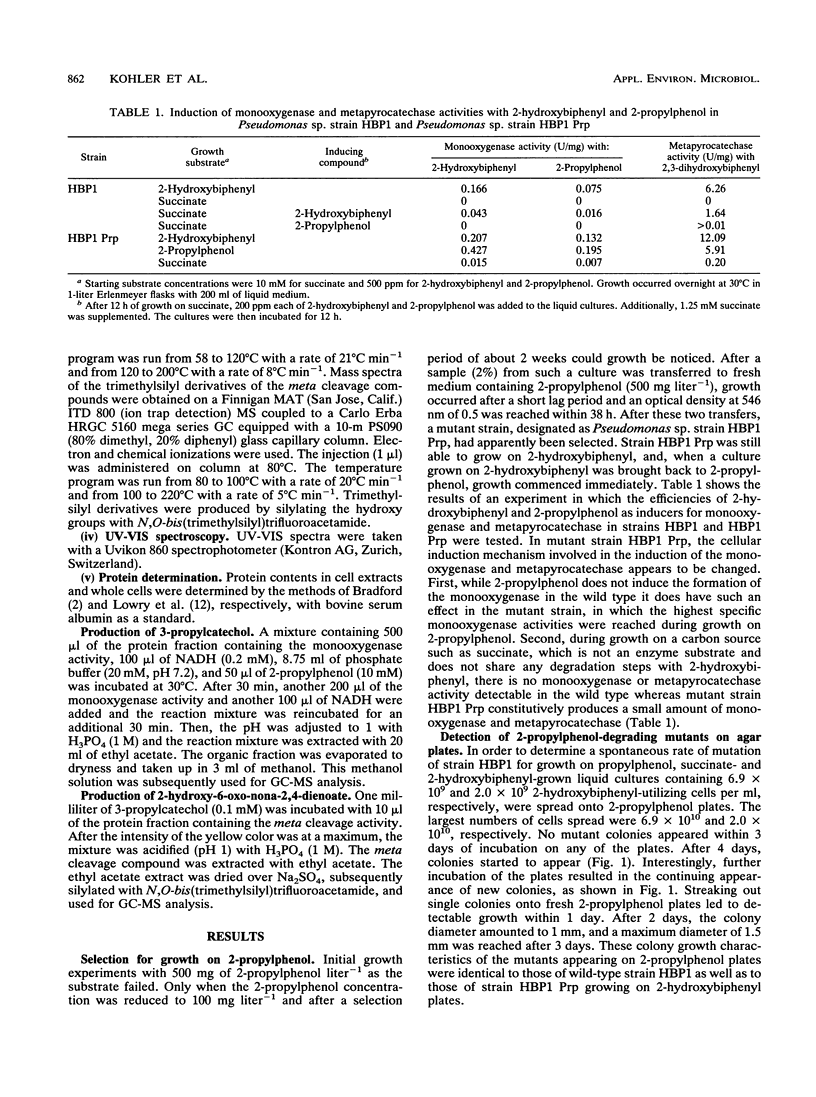
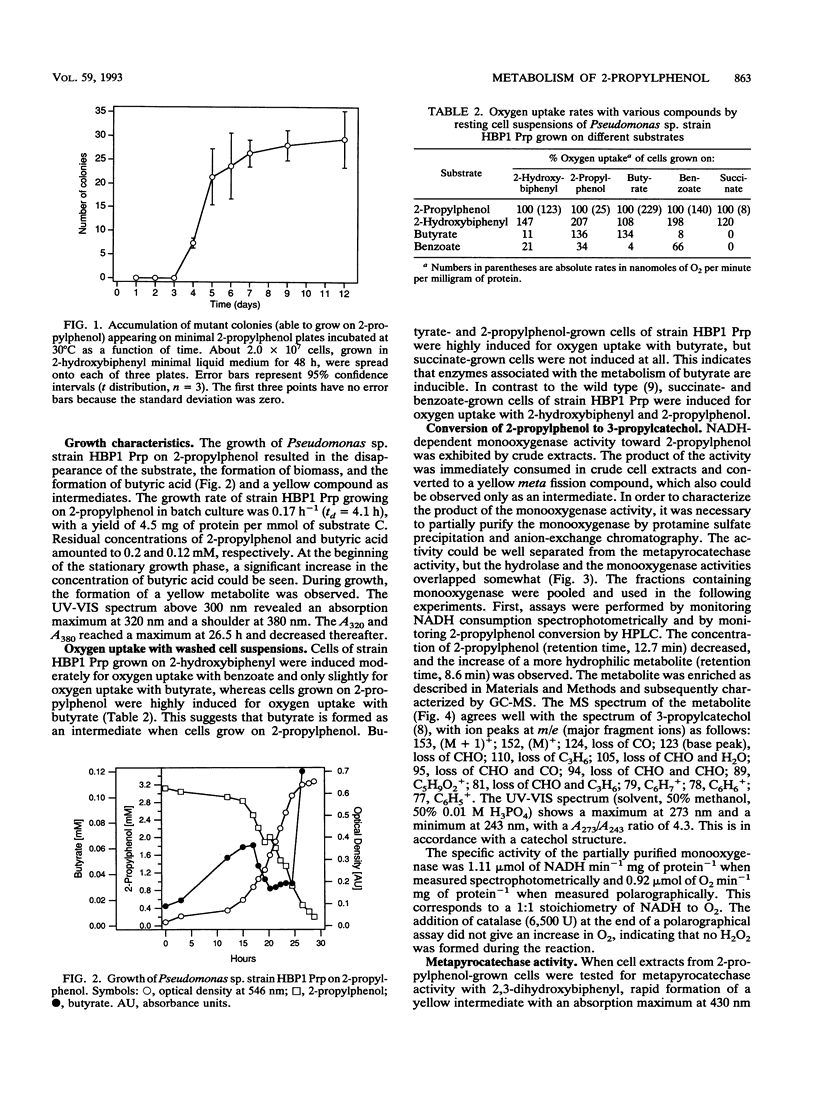
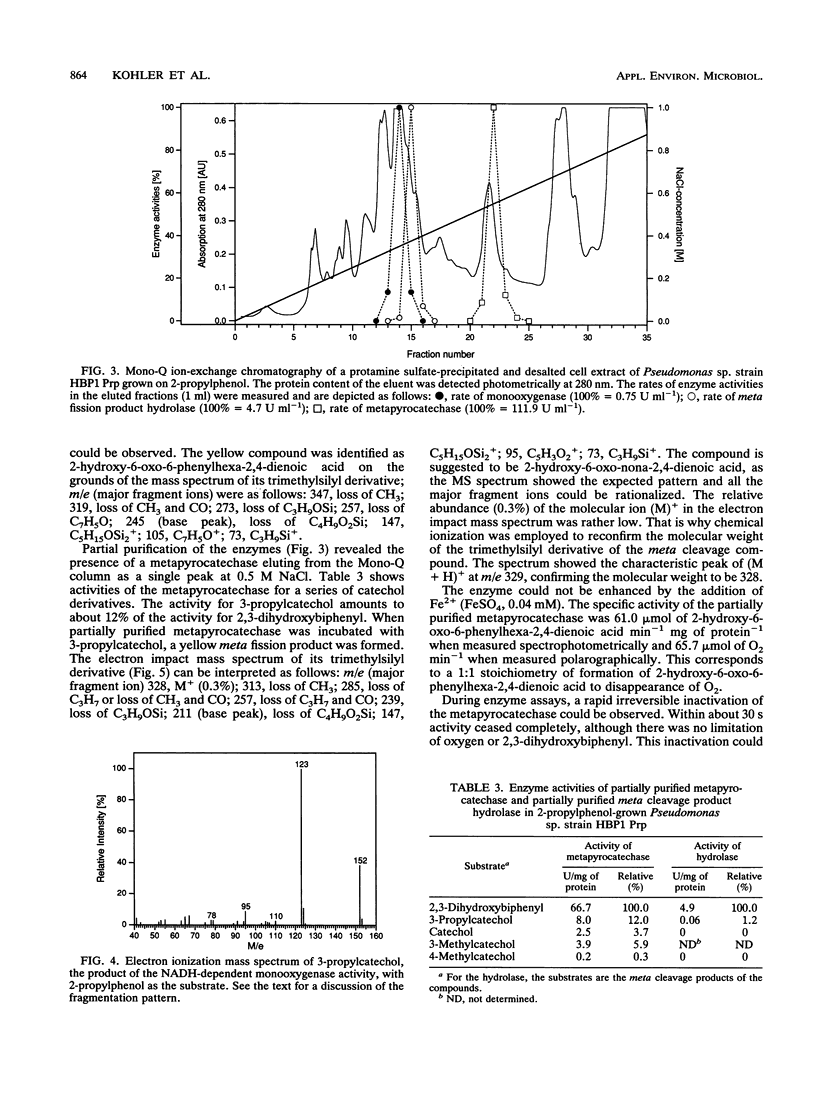
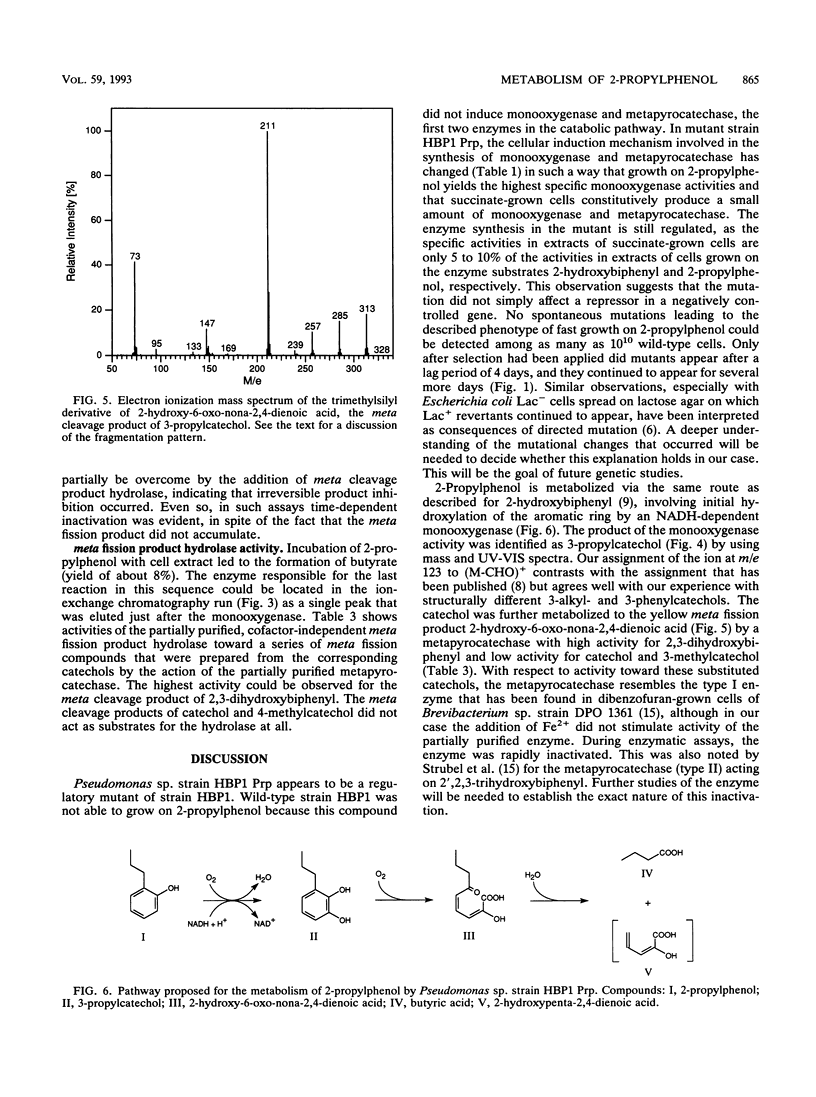
A mutant of Pseudomonas sp. strain HBP1, originally isolated on 2-hydroxybiphenyl, was selected for the ability to grow on 2-propylphenol as the sole carbon and energy source. In the mutant strain, which was designated as Pseudomonas sp. strain HBP1 Prp, the cellular induction mechanism involved in the synthesis of the NADH-dependent monooxygenase is changed. 2-Propylphenol, which is known to be a substrate of the monooxygenase, does not induce formation of the monooxygenase in the wild type but does have an induction effect in the mutant strain. Furthermore, in contrast to the wild type, mutant strain HBP1 Prp constitutively produces a small amount of monooxygenase and metapyrocatechase. The enzymes from strain HBP1 Prp catalyzing the first three steps in the degradation of 2-propylphenol--the NADH-dependent monooxygenase, the metapyrocatechase, and the meta fission product hydrolase--were partially purified, and their activities were measured. The product of the monooxygenase activity was identified by mass spectrometry as 3-propylcatechol. The metapyrocatechase used this compound as a substrate and produced a yellow meta fission product that was identified by mass spectrometry as 2-hydroxy-6-oxo-nona-2,4- dienoate. Butyrate could be detected as a product of the meta fission product hydrolase in crude cell extract of 2-propylphenol-grown cells, as well as an intermediate in culture broths during growth on 2-propylphenol. All three enzymes expressed highest activities for the metabolites of the degradation of 2-hydroxybiphenyl.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Ewers J., Rubio M. A., Knackmuss H. J., Freier-Schröder D. Bacterial metabolism of 2,6-xylenol. Appl Environ Microbiol. 1989 Nov;55(11):2904–2908. doi: 10.1128/aem.55.11.2904-2908.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L. Directed mutation: between unicorns and goats. J Bacteriol. 1992 Mar;174(6):1711–1716. doi: 10.1128/jb.174.6.1711-1716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higson F. K., Focht D. D. Bacterial metabolism of hydroxylated biphenyls. Appl Environ Microbiol. 1989 Apr;55(4):946–952. doi: 10.1128/aem.55.4.946-952.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jigami Y., Kawasaki Y., Omori T., Minoda Y. Coexistence of different pathways in the metabolism of n-propylbenzene by Pseudomonas sp. Appl Environ Microbiol. 1979 Nov;38(5):783–788. doi: 10.1128/aem.38.5.783-788.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler-Staub D., Kohler H. P. Microbial degradation of beta-chlorinated four-carbon aliphatic acids. J Bacteriol. 1989 Mar;171(3):1428–1434. doi: 10.1128/jb.171.3.1428-1434.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler H. P., Kohler-Staub D., Focht D. D. Degradation of 2-hydroxybiphenyl and 2,2'-dihydroxybiphenyl by Pseudomonas sp. strain HBP1. Appl Environ Microbiol. 1988 Nov;54(11):2683–2688. doi: 10.1128/aem.54.11.2683-2688.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz D. A., Chapman P. J. Catabolism of pseudocumene and 3-ethyltoluene by Pseudomonas putida (arvilla) mt-2: evidence for new functions of the TOL (pWWO) plasmid. J Bacteriol. 1981 Apr;146(1):179–191. doi: 10.1128/jb.146.1.179-191.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Smith M. R. The biodegradation of aromatic hydrocarbons by bacteria. Biodegradation. 1990;1(2-3):191–206. doi: 10.1007/BF00058836. [DOI] [PubMed] [Google Scholar]
- Strubel V., Engesser K. H., Fischer P., Knackmuss H. J. 3-(2-hydroxyphenyl)catechol as substrate for proximal meta ring cleavage in dibenzofuran degradation by Brevibacterium sp. strain DPO 1361. J Bacteriol. 1991 Mar;173(6):1932–1937. doi: 10.1128/jb.173.6.1932-1937.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


