Abstract
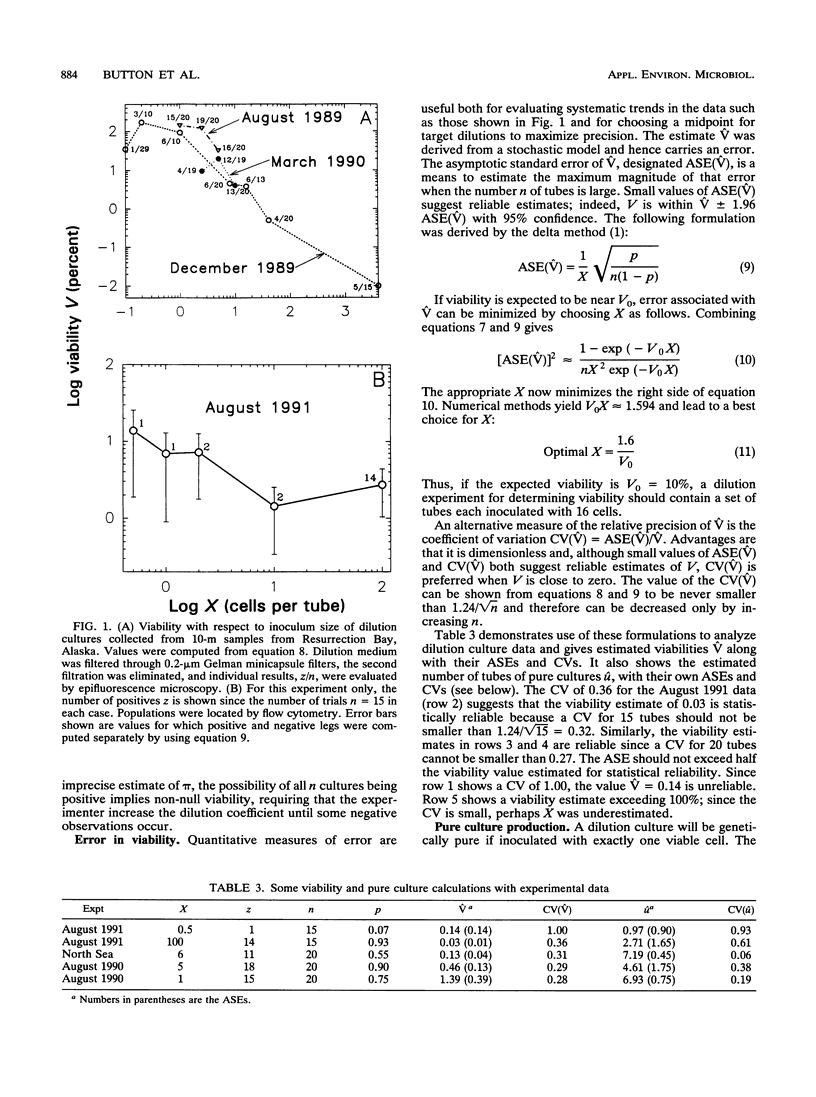
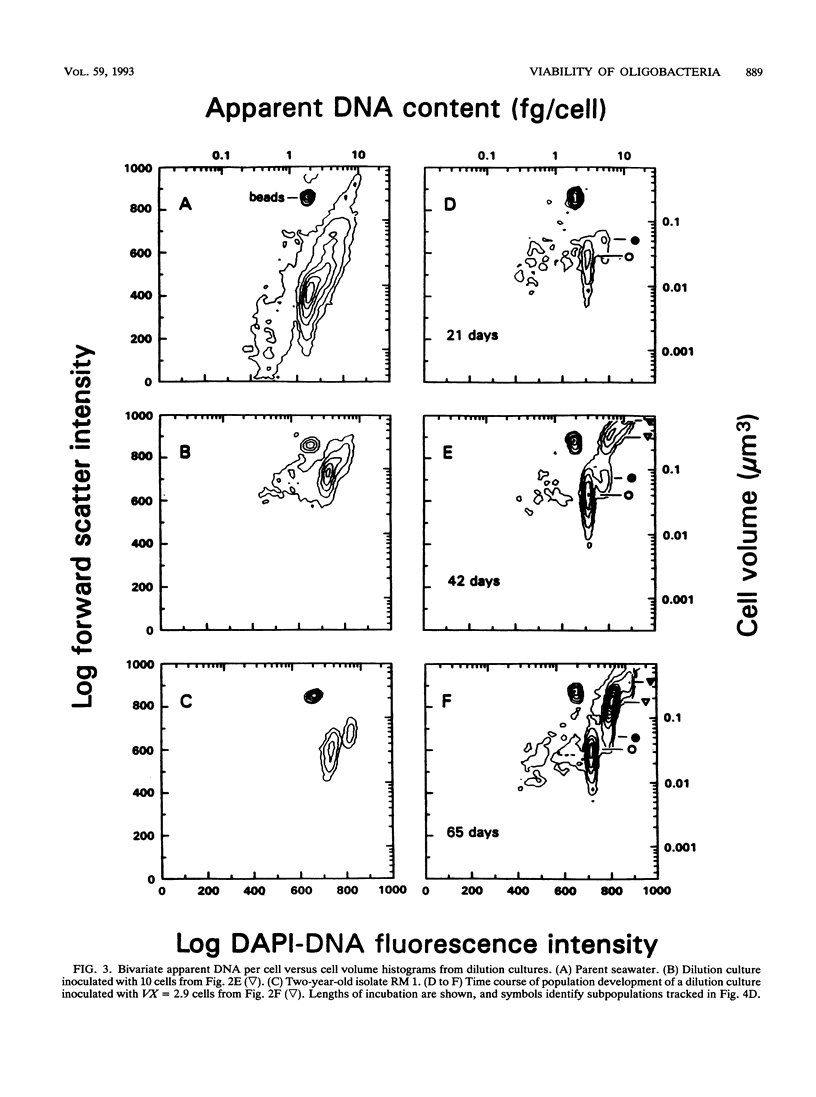
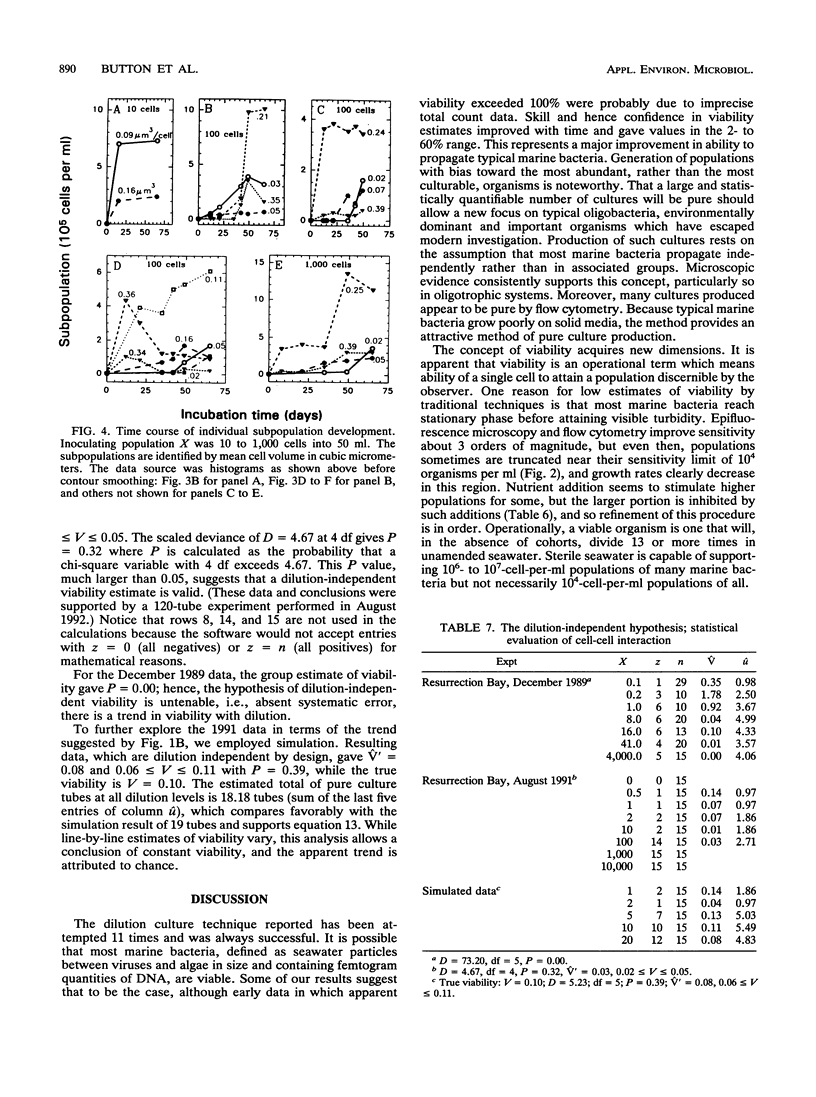
Dilution culture, a method for growing the typical small bacteria from natural aquatic assemblages, has been developed. Each of 11 experimental trials of the technique was successful. Populations are measured, diluted to a small and known number of cells, inoculated into unamended sterilized seawater, and examined three times for the presence of 104 or more cells per ml over a 9-week interval. Mean viability for assemblage members is obtained from the frequency of growth, and many of the cultures produced are pure. Statistical formulations for determining viability and the frequency of pure culture production are derived. Formulations for associated errors are derived as well. Computer simulations of experiments agreed with computed values within the expected error, which verified the formulations. These led to strategies for optimizing viability determinations and pure culture production. Viabilities were usually between 2 and 60% and decreased with >5 mg of amino acids per liter as carbon. In view of difficulties in growing marine oligobacteria, these high values are noteworthy. Significant differences in population characteristics during growth, observed by high-resolution flow cytometry, suggested substantial population diversity. Growth of total populations as well as of cytometry-resolved subpopulations sometimes were truncated at levels of near 104 cells per ml, showing that viable cells could escape detection. Viability is therefore defined as the ability to grow to that population; true viabilities could be even higher. Doubling times, based on whole populations as well as individual subpopulations, were in the 1-day to 1-week range. Data were examined for changes in viability with dilution suggesting cell-cell interactions, but none could be confirmed. The frequency of pure culture production can be adjusted by inoculum size if the viability is known. These apparently pure cultures produced retained the size and apparent DNA-content characteristic of the bulk of the organisms in the parent seawater. Three cultures are now available, two of which have been carried for 3 years. The method is thus seen as a useful step for improving our understanding of typical aquatic organisms.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benner R., Pakulski J. D., McCarthy M., Hedges J. I., Hatcher P. G. Bulk chemical characteristics of dissolved organic matter in the ocean. Science. 1992 Mar 20;255(5051):1561–1564. doi: 10.1126/science.255.5051.1561. [DOI] [PubMed] [Google Scholar]
- Bergh O., Børsheim K. Y., Bratbak G., Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989 Aug 10;340(6233):467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- Bratbak G., Heldal M., Norland S., Thingstad T. F. Viruses as partners in spring bloom microbial trophodynamics. Appl Environ Microbiol. 1990 May;56(5):1400–1405. doi: 10.1128/aem.56.5.1400-1405.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K. Biochemical basis for whole-cell uptake kinetics: specific affinity, oligotrophic capacity, and the meaning of the michaelis constant. Appl Environ Microbiol. 1991 Jul;57(7):2033–2038. doi: 10.1128/aem.57.7.2033-2038.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K., Robertson B. R. Kinetics of bacterial processes in natural aquatic systems based on biomass as determined by high-resolution flow cytometry. Cytometry. 1989 Sep;10(5):558–563. doi: 10.1002/cyto.990100511. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Ferguson R. L., Buckley E. N., Palumbo A. V. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984 Jan;47(1):49–55. doi: 10.1128/aem.47.1.49-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., McCallum K., Davis A. A. Novel major archaebacterial group from marine plankton. Nature. 1992 Mar 12;356(6365):148–149. doi: 10.1038/356148a0. [DOI] [PubMed] [Google Scholar]
- Haas C. N. Estimation of microbial densities from dilution count experiments. Appl Environ Microbiol. 1989 Aug;55(8):1934–1942. doi: 10.1128/aem.55.8.1934-1942.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. Distribution of viable marine bacteria in neritic seawater around Japan. Can J Microbiol. 1980 Mar;26(3):318–323. doi: 10.1139/m80-052. [DOI] [PubMed] [Google Scholar]
- Oliver J. D., Nilsson L., Kjelleberg S. Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl Environ Microbiol. 1991 Sep;57(9):2640–2644. doi: 10.1128/aem.57.9.2640-2644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H., Jiang S. C., Rose J. B. Concentration of viruses and dissolved DNA from aquatic environments by vortex flow filtration. Appl Environ Microbiol. 1991 Aug;57(8):2197–2204. doi: 10.1128/aem.57.8.2197-2204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. F., Parton R., Wardlaw A. C. Growth and survival of Bordetella bronchiseptica in natural waters and in buffered saline without added nutrients. Appl Environ Microbiol. 1991 Apr;57(4):1202–1206. doi: 10.1128/aem.57.4.1202-1206.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. R., Button D. K. Characterizing aquatic bacteria according to population, cell size, and apparent DNA content by flow cytometry. Cytometry. 1989 Jan;10(1):70–76. doi: 10.1002/cyto.990100112. [DOI] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Metabolic activity of bacterial cells enumerated by direct viable count. Appl Environ Microbiol. 1987 Dec;53(12):2889–2893. doi: 10.1128/aem.53.12.2889-2893.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle C. A., Chan A. M., Cottrell M. T. Use of ultrafiltration to isolate viruses from seawater which are pathogens of marine phytoplankton. Appl Environ Microbiol. 1991 Mar;57(3):721–726. doi: 10.1128/aem.57.3.721-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



