Abstract
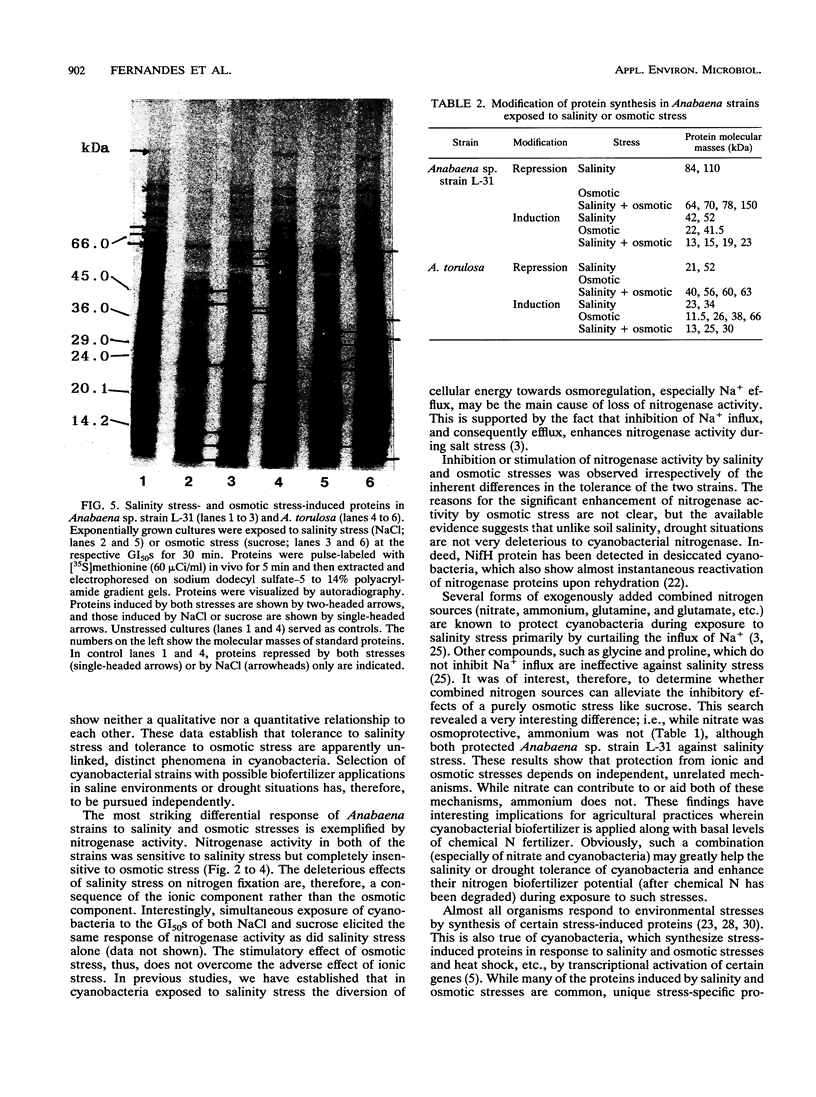
Two nitrogen-fixing Anabaena strains were found to be differentially tolerant to salinity and osmotic stresses. Anabaena torulosa, a brackish-water, salt-tolerant strain, was relatively osmosensitive. Anabaena sp. strain L-31, a freshwater, salt-sensitive strain, on the other hand, displayed significant osmotolerance. Salinity and osmotic stresses affected nitrogenase activity differently. Nitrogen fixation in both of the strains was severely inhibited by the ionic, but not by the osmotic, component of salinity stress. Such differential sensitivity of diazotrophy to salinity-osmotic stresses was observed irrespective of the inherent tolerance of the two strains to salt-osmotic stress. Exogenously added ammonium conferred significant protection against salinity stress but was ineffective against osmotic stress. Salinity and osmotic stresses also affected stress-induced gene expression differently. Synthesis of several proteins was repressed by salinity stress but not by equivalent or higher osmotic stress. Salinity and osmotic stresses induced many common proteins. In addition, unique salt stress- or osmotic stress-specific proteins were also induced in both strains, indicating differential regulation of protein synthesis by the two stresses. These data show that cyanobacterial sensitivity and responses to salinity and osmotic stresses are distinct, independent phenomena.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte S. K., Bhagwat A. A. Salinity-stress-induced proteins in two nitrogen-fixing Anabaena strains differentially tolerant to salt. J Bacteriol. 1989 Feb;171(2):909–915. doi: 10.1128/jb.171.2.909-915.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte S. K., Haselkorn R. Cloning of salinity stress-induced genes from the salt-tolerant nitrogen-fixing cyanobacterium Anabaena torulosa. Plant Mol Biol. 1990 Nov;15(5):723–733. doi: 10.1007/BF00016122. [DOI] [PubMed] [Google Scholar]
- Apte S. K., Reddy B. R., Thomas J. Relationship between Sodium Influx and Salt Tolerance of Nitrogen-Fixing Cyanobacteria. Appl Environ Microbiol. 1987 Aug;53(8):1934–1939. doi: 10.1128/aem.53.8.1934-1939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte S. K., Thomas J. Membrane electrogenesis and sodium transport in filamentous nitrogen-fixing cyanobacteria. Eur J Biochem. 1986 Jan 15;154(2):395–401. doi: 10.1111/j.1432-1033.1986.tb09411.x. [DOI] [PubMed] [Google Scholar]
- Bhagwat A. A., Apte S. K. Comparative analysis of proteins induced by heat shock, salinity, and osmotic stress in the nitrogen-fixing cyanobacterium Anabaena sp. strain L-31. J Bacteriol. 1989 Sep;171(9):5187–5189. doi: 10.1128/jb.171.9.5187-5189.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Mehlhorn R. J., Packer L. Studies of osmoregulation in salt adaptation of cyanobacteria with ESR spin-probe techniques. Proc Natl Acad Sci U S A. 1983 May;80(9):2599–2602. doi: 10.1073/pnas.80.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Tel-Or E. Salt adaptation of the cyanobacterium synechococcus 6311 growing in a continuous culture (turbidostat). Plant Physiol. 1984 Jan;74(1):183–185. doi: 10.1104/pp.74.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitzka L. J., Demmerle S., Mackay M. A., Norton R. S. Carbon-13 nuclear magnetic resonance study of osmoregulation in a blue-green alga. Science. 1980 Nov 7;210(4470):650–651. doi: 10.1126/science.210.4470.650. [DOI] [PubMed] [Google Scholar]
- Claes B., Dekeyser R., Villarroel R., Van den Bulcke M., Bauw G., Van Montagu M., Caplan A. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell. 1990 Jan;2(1):19–27. doi: 10.1105/tpc.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar J. Nucleotide sequence of the osmoregulatory proU operon of Escherichia coli. J Bacteriol. 1989 Apr;171(4):1923–1931. doi: 10.1128/jb.171.4.1923-1931.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., Jones J. H., Yopp J. H., Tindall D. R., Schmid W. E. Ion metabolism in a halophilic blue-green alga, Aphanothece halophytica. Arch Microbiol. 1976 Dec 1;111(1-2):145–149. doi: 10.1007/BF00446561. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Ramagopal S. Salinity stress induced tissue-specific proteins in barley seedlings. Plant Physiol. 1987 Jun;84(2):324–331. doi: 10.1104/pp.84.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez R. M., Prince W. S., Bremer E., Villarejo M. In vitro reconstitution of osmoregulated expression of proU of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1153–1157. doi: 10.1073/pnas.86.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B. R., Apte S. K., Thomas J. Enhancement of cyanobacterial salt tolerance by combined nitrogen. Plant Physiol. 1989 Jan;89(1):204–210. doi: 10.1104/pp.89.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Nelson D. E., Kuhn D., Hasegawa P. M., Bressan R. A. Molecular Cloning of Osmotin and Regulation of Its Expression by ABA and Adaptation to Low Water Potential. Plant Physiol. 1989 Jul;90(3):1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector M. P., Aliabadi Z., Gonzalez T., Foster J. W. Global control in Salmonella typhimurium: two-dimensional electrophoretic analysis of starvation-, anaerobiosis-, and heat shock-inducible proteins. J Bacteriol. 1986 Oct;168(1):420–424. doi: 10.1128/jb.168.1.420-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. Absence of the pigments of photosystem II of photosynthesis in heterocysts of a blue-green alga. Nature. 1970 Oct 10;228(5267):181–183. doi: 10.1038/228181b0. [DOI] [PubMed] [Google Scholar]
- Weretilnyk E. A., Hanson A. D. Molecular cloning of a plant betaine-aldehyde dehydrogenase, an enzyme implicated in adaptation to salinity and drought. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2745–2749. doi: 10.1073/pnas.87.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]



