Abstract
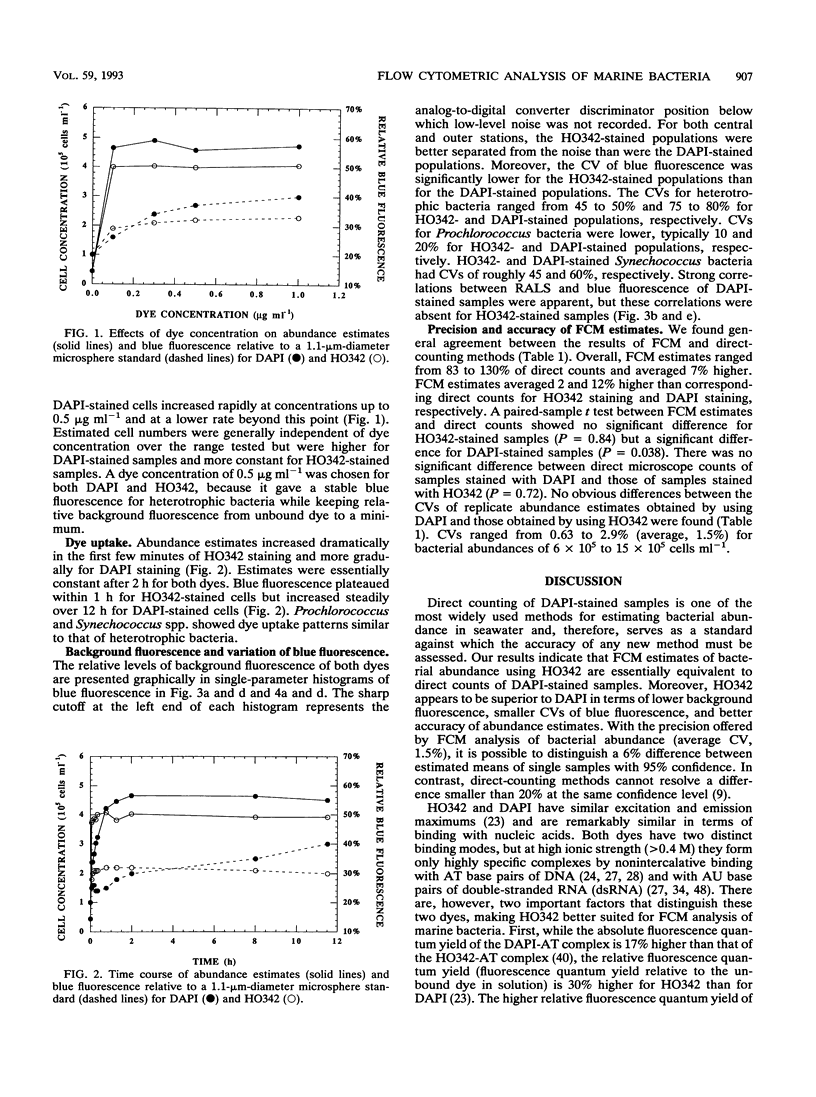
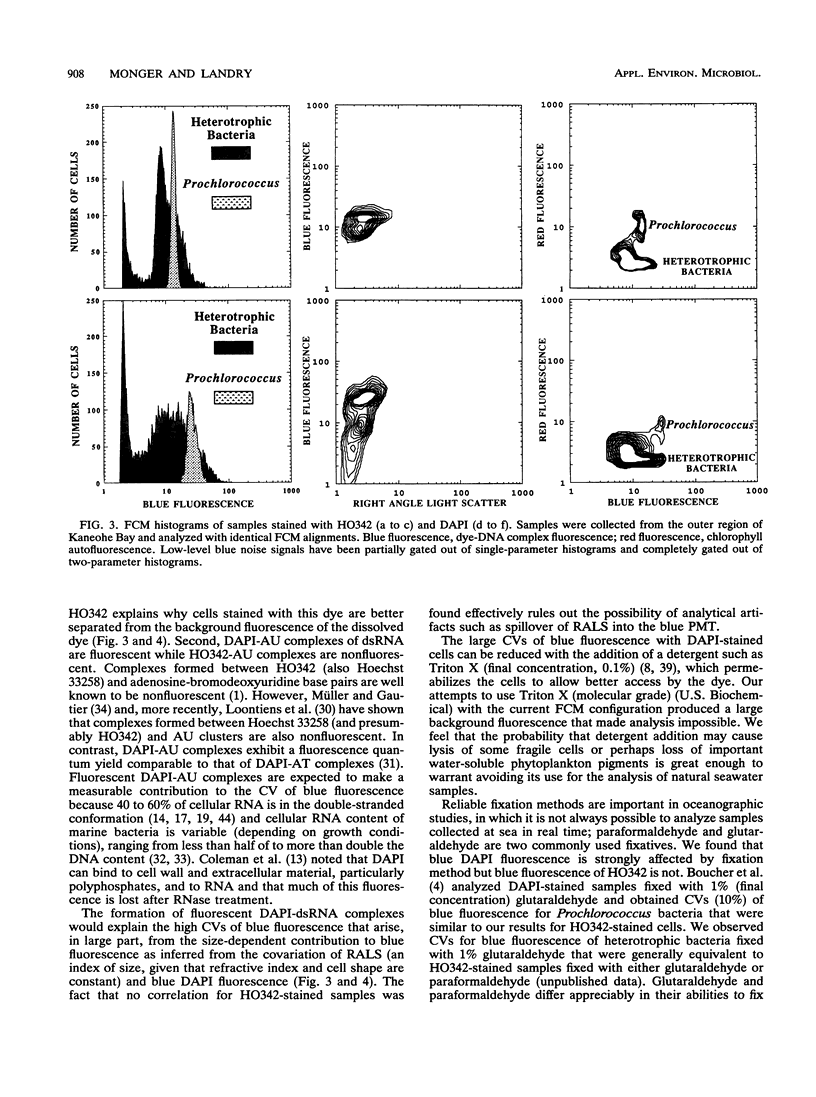
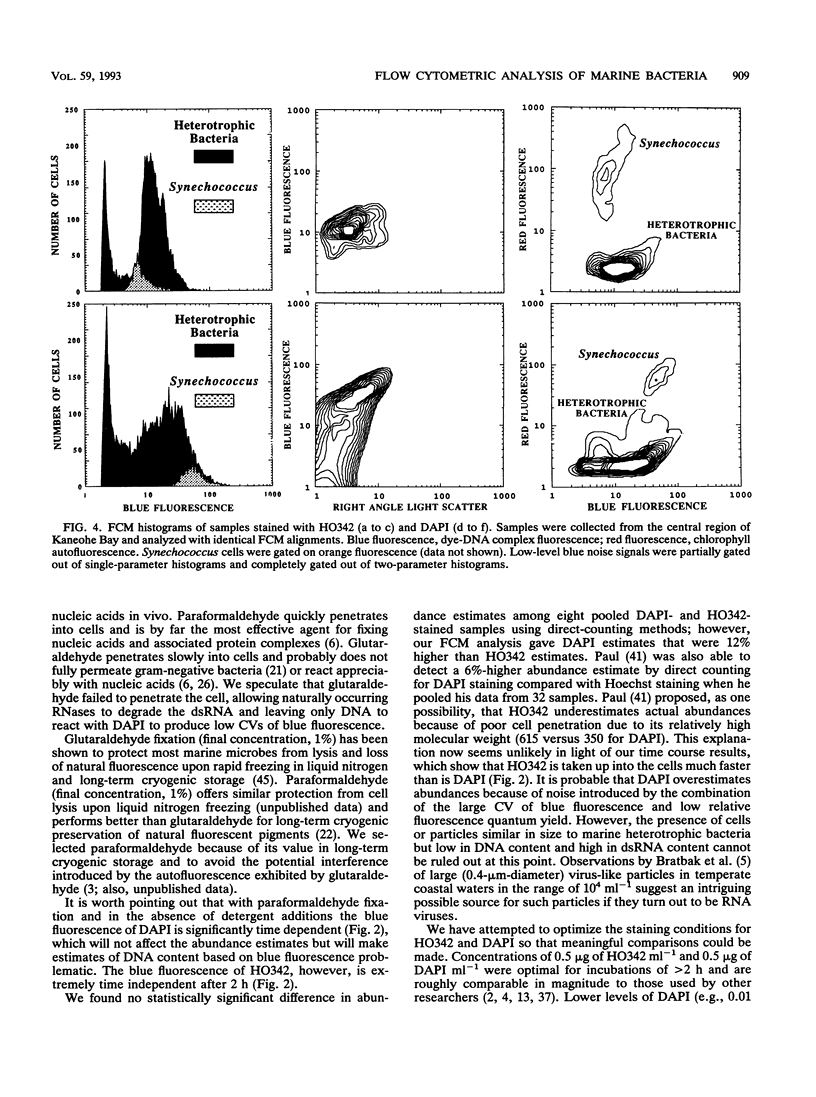
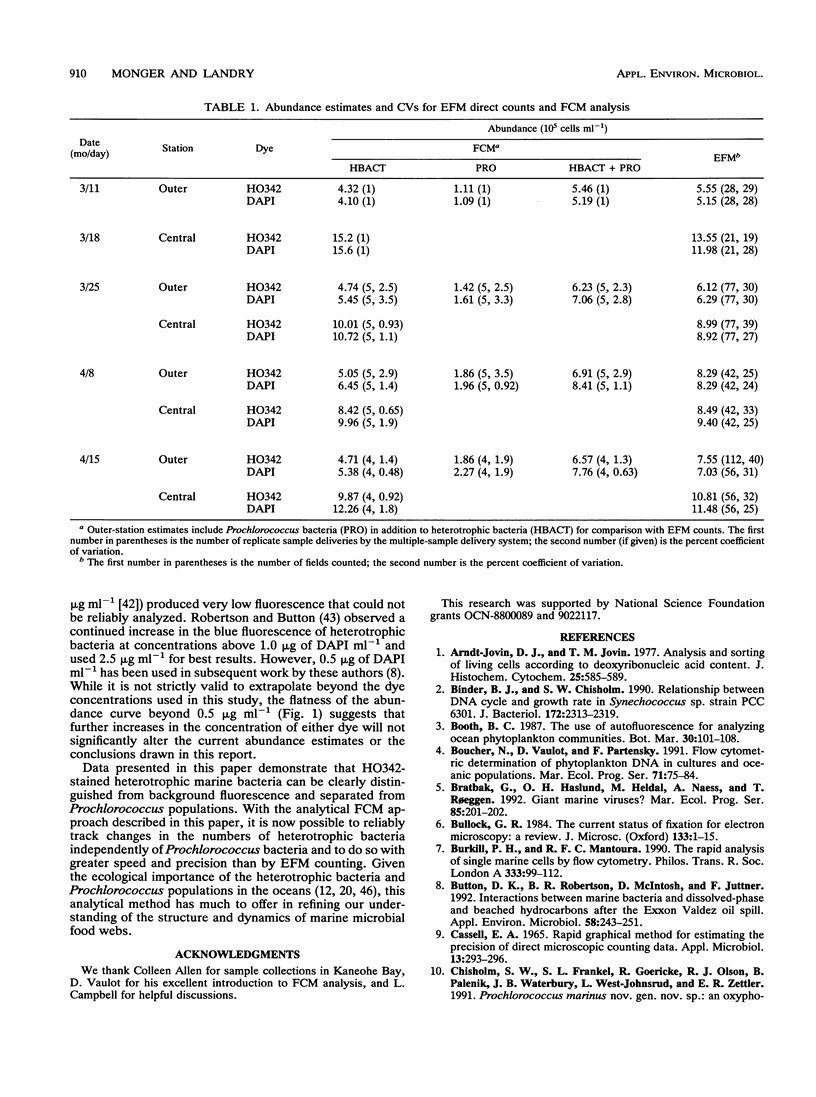
We investigated the accuracy and precision of flow cytometric (FCM) estimates of bacterial abundances using 4′, 6-diamidino-2-phenylindole (DAPI) and Hoechst 33342 (HO342, a bisbenzamide derivative) on paraformaldehyde-fixed seawater samples collected from two stations near Oahu, Hawaii. The accuracy of FCM estimates was assessed against direct counts by using epifluorescence microscopy. DAPI and HO342 differ in two aspects of their chemistry that make HO342 better suited for staining marine heterotrophic bacteria for FCM analysis. These differences are most important in studies of open-ocean ecosystems that require dual-beam FCM analysis to clearly separate heterotrophic bacterial populations from populations of photosynthetic Prochlorococcus spp. Bacterial populations were easier to distinguish from background fluorescence when stained with HO342 than when stained with DAPI, because HO342 has a higher relative fluorescence quantum yield. A substantially higher coefficient of variation of blue fluorescence, which was probably due to fluorescent complexes formed by DAPI with double-stranded RNA, was observed for DAPI-stained populations. FCM estimates averaged 2.0 and 12% higher than corresponding epifluorescence microscopy direct counts for HO342 and DAPI-stained samples, respectively. A paired-sample t test between FCM estimates and direct counts found no significant difference for HO342-stained samples but a significant difference for DAPI-stained samples. Coefficients of variation of replicate FCM abundance estimates ranged from 0.63 to 2.9% (average, 1.5%) for natural bacterial concentrations of 6 × 105 to 15 × 105 cells ml-1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Jovin T. M. Analysis and sorting of living cells according to deoxyribonucleic acid content. J Histochem Cytochem. 1977 Jul;25(7):585–589. doi: 10.1177/25.7.70450. [DOI] [PubMed] [Google Scholar]
- Binder B. J., Chisholm S. W. Relationship between DNA cycle and growth rate in Synechococcus sp. strain PCC 6301. J Bacteriol. 1990 May;172(5):2313–2319. doi: 10.1128/jb.172.5.2313-2319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K., Robertson B. R., McIntosh D., Jüttner F. Interactions between marine bacteria and dissolved-phase and beached hydrocarbons after the Exxon Valdez oil spill. Appl Environ Microbiol. 1992 Jan;58(1):243–251. doi: 10.1128/aem.58.1.243-251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASSELL E. A. RAPID GRAPHICAL METHOD FOR ESTIMATING THE PRECISION OF DIRECT MICROSCOPIC COUNTING DATA. Appl Microbiol. 1965 May;13:293–296. doi: 10.1128/am.13.3.293-296.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman A. W., Maguire M. J., Coleman J. R. Mithramycin- and 4'-6-diamidino-2-phenylindole (DAPI)-DNA staining for fluorescence microspectrophotometric measurement of DNA in nuclei, plastids, and virus particles. J Histochem Cytochem. 1981 Aug;29(8):959–968. doi: 10.1177/29.8.6168681. [DOI] [PubMed] [Google Scholar]
- Cotter R. I., McPhie P., Gratzer W. B. Internal organization of the ribosome. Nature. 1967 Dec 2;216(5118):864–868. doi: 10.1038/216864a0. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Conformation of RNA in situ as studied by acridine orange staining and automated cytofluorometry. Exp Cell Res. 1975 Oct 1;95(1):143–153. doi: 10.1016/0014-4827(75)90619-9. [DOI] [PubMed] [Google Scholar]
- Doty P., Boedtker H., Fresco J. R., Haselkorn R., Litt M. SECONDARY STRUCTURE IN RIBONUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1959 Apr;45(4):482–499. doi: 10.1073/pnas.45.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman S. P., Scott E. M., Russell A. D. Antimicrobial activity, uses and mechanism of action of glutaraldehyde. J Appl Bacteriol. 1980 Apr;48(2):161–190. doi: 10.1111/j.1365-2672.1980.tb01217.x. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J. Interactions of nucleic acids with fluorescent dyes: spectral properties of condensed complexes. J Histochem Cytochem. 1990 Sep;38(9):1323–1329. doi: 10.1177/38.9.1696951. [DOI] [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Fluorescent complexes of DNA with DAPI 4',6-diamidine-2-phenyl indole.2HCl or DCI 4',6-dicarboxyamide-2-phenyl indole. Nucleic Acids Res. 1978 Oct;5(10):3775–3799. doi: 10.1093/nar/5.10.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenburg W. G. Glutaraldehyde nonfixation of isolated viral and yeast RNAs. J Histochem Cytochem. 1980 Apr;28(4):311–315. doi: 10.1177/28.4.6768793. [DOI] [PubMed] [Google Scholar]
- Larsen T. A., Goodsell D. S., Cascio D., Grzeskowiak K., Dickerson R. E. The structure of DAPI bound to DNA. J Biomol Struct Dyn. 1989 Dec;7(3):477–491. doi: 10.1080/07391102.1989.10508505. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Stetten G. Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J Histochem Cytochem. 1976 Jan;24(1):24–33. doi: 10.1177/24.1.943439. [DOI] [PubMed] [Google Scholar]
- Loewe H., Urbanietz J. Basisch substituierte 2,6-Bis-benzimidazolderivate, eine neue chemotherapeutisch aktive Körperklasse. Arzneimittelforschung. 1974 Dec;24(12):1927–1933. [PubMed] [Google Scholar]
- Loontiens F. G., McLaughlin L. W., Diekmann S., Clegg R. M. Binding of Hoechst 33258 and 4',6'-diamidino-2-phenylindole to self-complementary decadeoxynucleotides with modified exocyclic base substituents. Biochemistry. 1991 Jan 8;30(1):182–189. doi: 10.1021/bi00215a027. [DOI] [PubMed] [Google Scholar]
- Manzini G., Xodo L., Barcellona M. L., Quadrifoglio F. Interaction of DAPI with double-stranded ribonucleic acids. Nucleic Acids Res. 1985 Dec 20;13(24):8955–8967. doi: 10.1093/nar/13.24.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer C. L., Morita R. Y. Effect of growth rate and starvation-survival on cellular DNA, RNA, and protein of a psychrophilic marine bacterium. Appl Environ Microbiol. 1989 Oct;55(10):2710–2716. doi: 10.1128/aem.55.10.2710-2716.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Gautier F. Interactions of heteroaromatic compounds with nucleic acids. A - T-specific non-intercalating DNA ligands. Eur J Biochem. 1975 Jun;54(2):385–394. doi: 10.1111/j.1432-1033.1975.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Otto F. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. Methods Cell Biol. 1990;33:105–110. doi: 10.1016/s0091-679x(08)60516-6. [DOI] [PubMed] [Google Scholar]
- Otto F., Tsou K. C. A comparative study of DAPI, DIPI, and Hoechst 33258 and 33342 as chromosomal DNA stains. Stain Technol. 1985 Jan;60(1):7–11. doi: 10.3109/10520298509113885. [DOI] [PubMed] [Google Scholar]
- Paul J. H. Use of hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol. 1982 Apr;43(4):939–944. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. R., Button D. K. Characterizing aquatic bacteria according to population, cell size, and apparent DNA content by flow cytometry. Cytometry. 1989 Jan;10(1):70–76. doi: 10.1002/cyto.990100112. [DOI] [PubMed] [Google Scholar]
- Traganos F., Darzynkiewicz Z., Sharpless T., Melamed M. R. Simultaneous staining of ribonucleic and deoxyribonucleic acids in unfixed cells using acridine orange in a flow cytofluorometric system. J Histochem Cytochem. 1977 Jan;25(1):46–56. doi: 10.1177/25.1.64567. [DOI] [PubMed] [Google Scholar]
- Vaulot D., Courties C., Partensky F. A simple method to preserve oceanic phytoplankton for flow cytometric analyses. Cytometry. 1989 Sep;10(5):629–635. doi: 10.1002/cyto.990100519. [DOI] [PubMed] [Google Scholar]


