Abstract
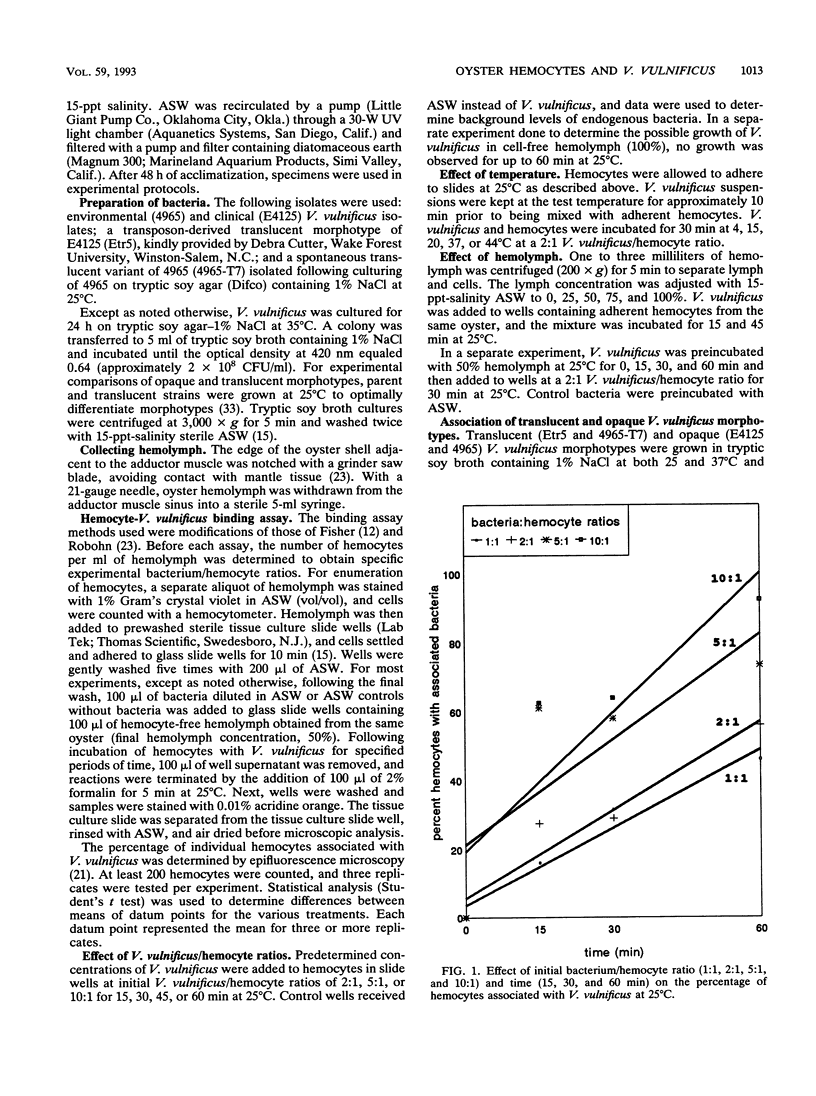
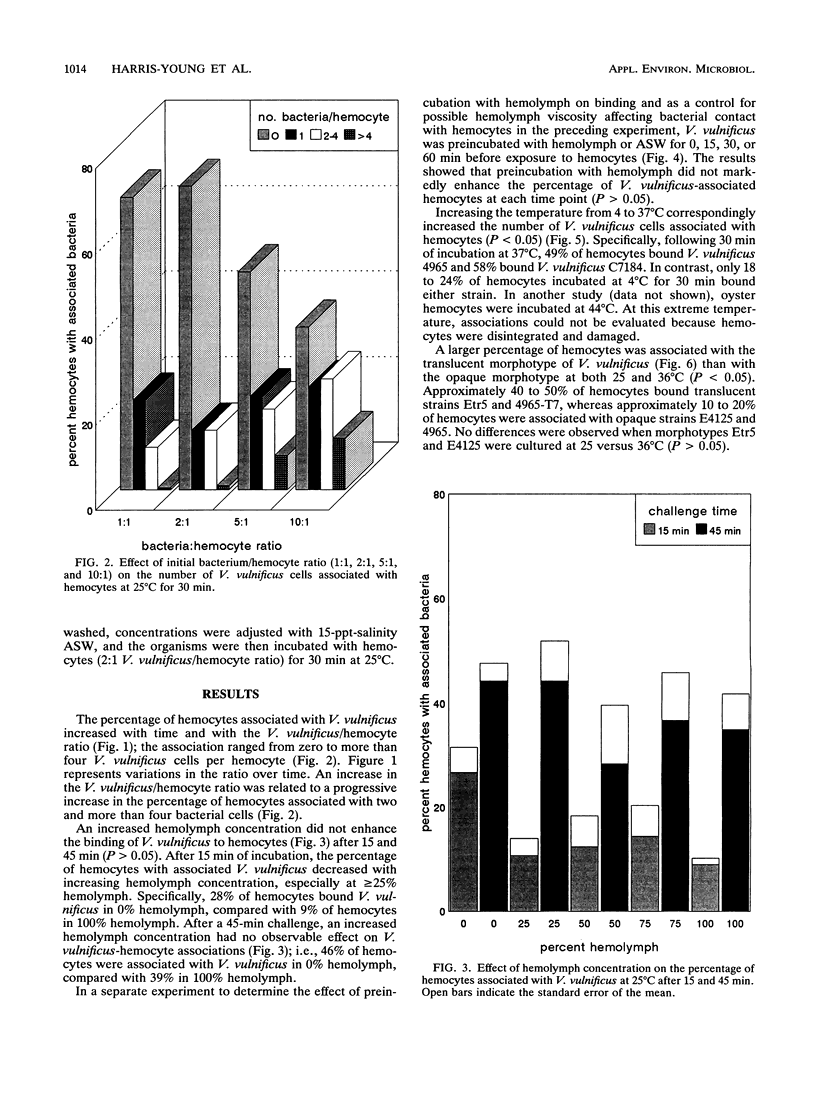
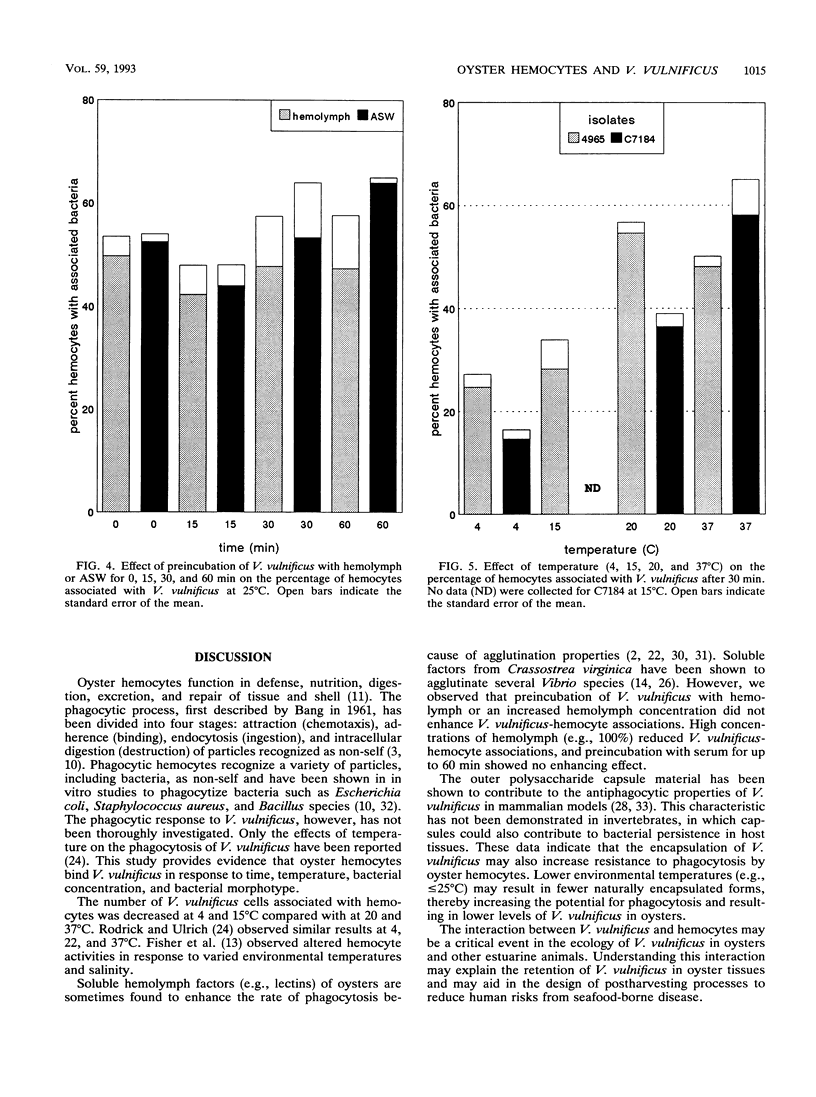
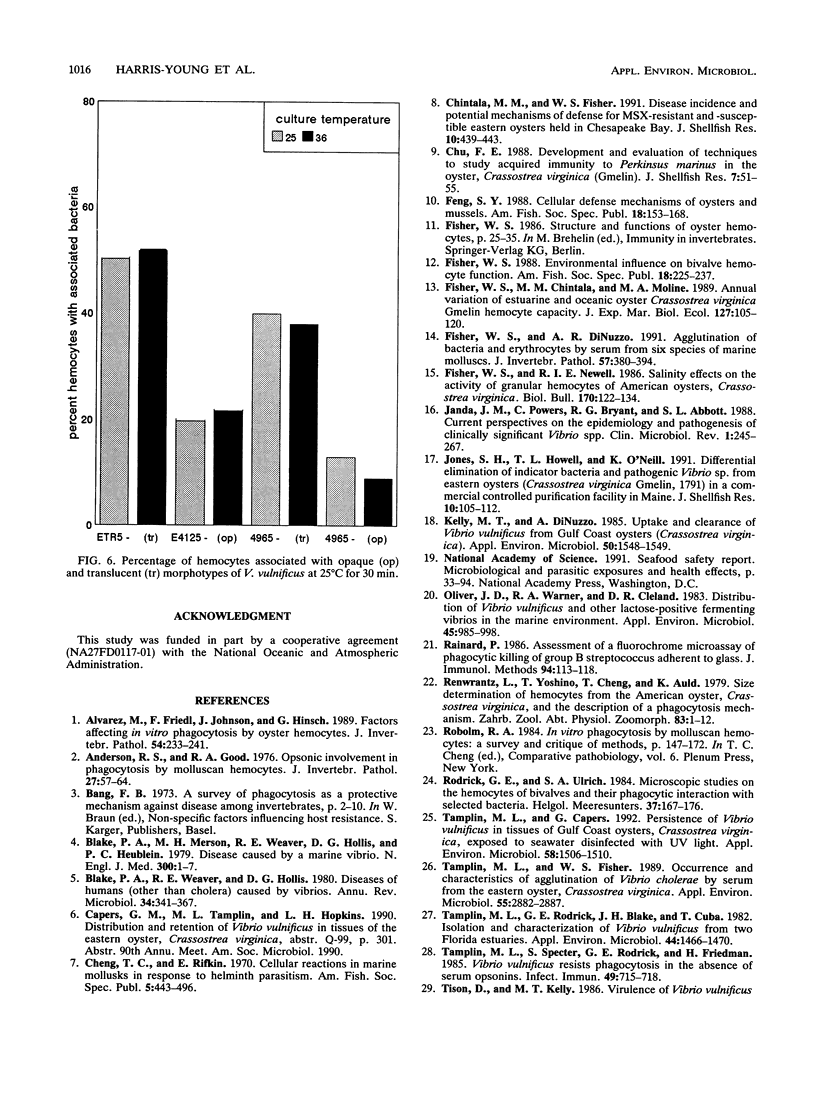
Vibrio vulnificus is a naturally occurring marine bacterium that causes invasive disease of immunocompromised humans following the consumption of raw oysters. It is a component of the natural microbiota of Gulf Coast estuaries and has been found to inhabit tissues of oysters, Crassostrea virginica (Gmelin 1791). The interaction of V. vulnificus with oyster host defenses has not been reported in detail. We examined the interaction of V. vulnificus with phagocytic oyster hemocytes as a function of time, temperature, bacterial concentration, pretreatment with hemolymph, and V. vulnificus translucent and opaque colony morphotypes. Within these experimental parameters, the results showed that the association of V. vulnificus with hemocytes increased with time, temperature, and initial V. vulnificus/hemocyte ratio. Pretreatment of V. vulnificus with serum or an increased serum concentration did not enhance V. vulnificus-hemocyte associations, a result suggesting the absence of opsonic activity. More than 50% of hemocytes bound the translucent, avirulent morphotype, whereas 10 to 20% were associated with the opaque, virulent form, a result indicating that the degree of encapsulation was related to resistance to phagocytosis, as previously described for mammalian phagocytes. Understanding these cellular interactions may, in part, explain the persistence of V. vulnificus in oyster tissues and the ecology of V. vulnificus in estuarine environments.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. S., Good R. A. Opsonic involvement in phagocytosis by mollusk hemocytes. J Invertebr Pathol. 1976 Jan;27(1):57–64. doi: 10.1016/0022-2011(76)90028-8. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Merson M. H., Weaver R. E., Hollis D. G., Heublein P. C. Disease caused by a marine Vibrio. Clinical characteristics and epidemiology. N Engl J Med. 1979 Jan 4;300(1):1–5. doi: 10.1056/NEJM197901043000101. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Weaver R. E., Hollis D. G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Fisher W. S., DiNuzzo A. R. Agglutination of bacteria and erythrocytes by serum from six species of marine molluscs. J Invertebr Pathol. 1991 May;57(3):380–394. doi: 10.1016/0022-2011(91)90142-d. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Powers C., Bryant R. G., Abbott S. L. Current perspectives on the epidemiology and pathogenesis of clinically significant Vibrio spp. Clin Microbiol Rev. 1988 Jul;1(3):245–267. doi: 10.1128/cmr.1.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T., Dinuzzo A. Uptake and clearance of Vibrio vulnificus from Gulf coast oysters (Crassostrea virginica). Appl Environ Microbiol. 1985 Dec;50(6):1548–1549. doi: 10.1128/aem.50.6.1548-1549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Warner R. A., Cleland D. R. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl Environ Microbiol. 1983 Mar;45(3):985–998. doi: 10.1128/aem.45.3.985-998.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainard P. Assessment by a fluorochrome microassay of phagocytic killing of group B streptococci adherent to glass. J Immunol Methods. 1986 Nov 20;94(1-2):113–118. doi: 10.1016/0022-1759(86)90222-x. [DOI] [PubMed] [Google Scholar]
- Tamplin M. L., Capers G. M. Persistence of Vibrio vulnificus in tissues of Gulf Coast oysters, Crassostrea virginica, exposed to seawater disinfected with UV light. Appl Environ Microbiol. 1992 May;58(5):1506–1510. doi: 10.1128/aem.58.5.1506-1510.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin M. L., Fisher W. S. Occurrence and characteristics of agglutination of Vibrio cholerae by serum from the eastern oyster, Crassostrea virginica. Appl Environ Microbiol. 1989 Nov;55(11):2882–2887. doi: 10.1128/aem.55.11.2882-2887.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin M. L., Specter S., Rodrick G. E., Friedman H. Vibrio vulnificus resists phagocytosis in the absence of serum opsonins. Infect Immun. 1985 Sep;49(3):715–718. doi: 10.1128/iai.49.3.715-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin M., Rodrick G. E., Blake N. J., Cuba T. Isolation and characterization of Vibrio vulnificus from two Florida estuaries. Appl Environ Microbiol. 1982 Dec;44(6):1466–1470. doi: 10.1128/aem.44.6.1466-1470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp M. R. Hemagglutinin in the blood of the oyster Crassostrea virginica. J Invertebr Pathol. 1966 Dec;8(4):478–484. doi: 10.1016/0022-2011(66)90074-7. [DOI] [PubMed] [Google Scholar]
- Weinheimer P. F., Acton R. T., Evans E. E. Attempt to induce a bactericidal response in the oyster. J Bacteriol. 1969 Jan;97(1):462–463. doi: 10.1128/jb.97.1.462-463.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


