Abstract
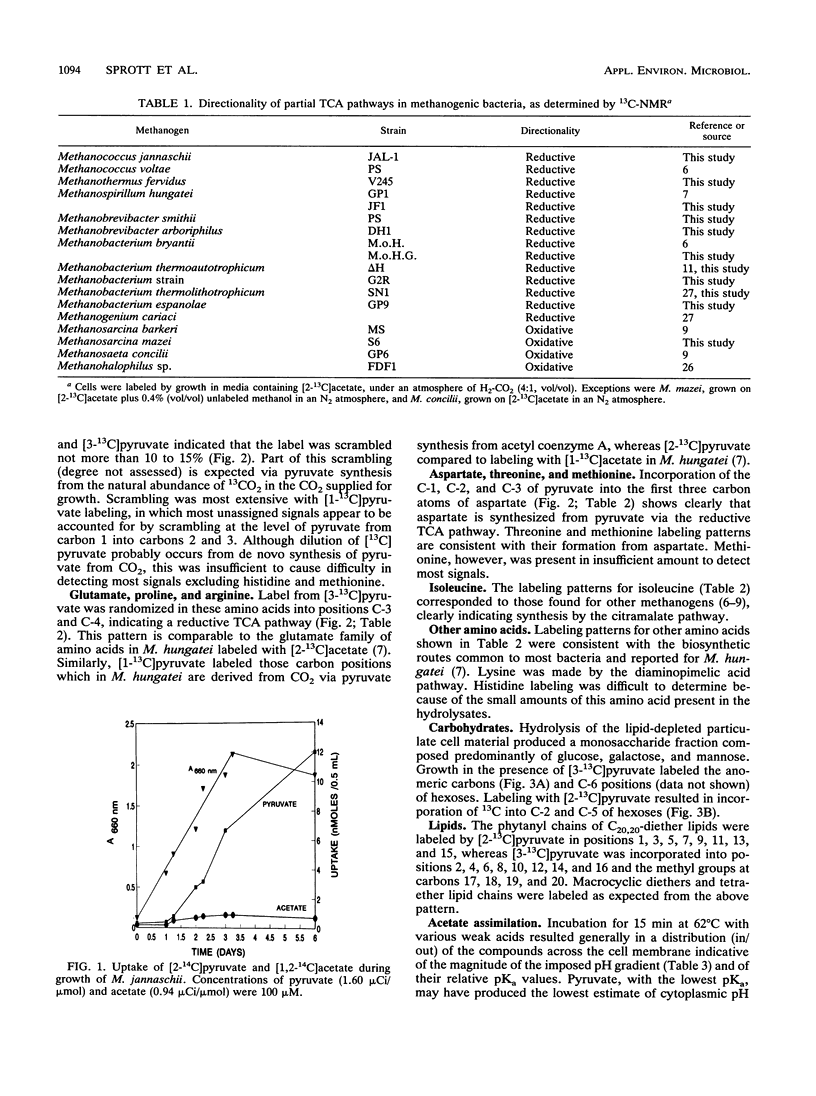
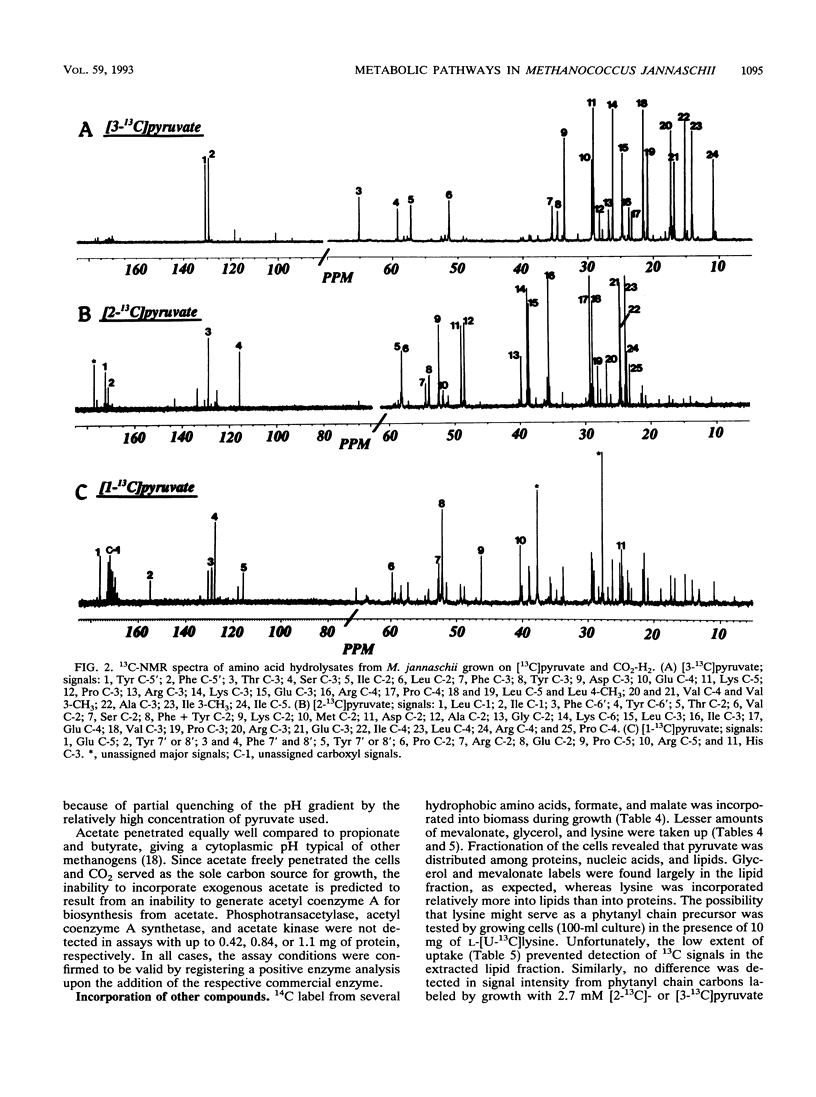
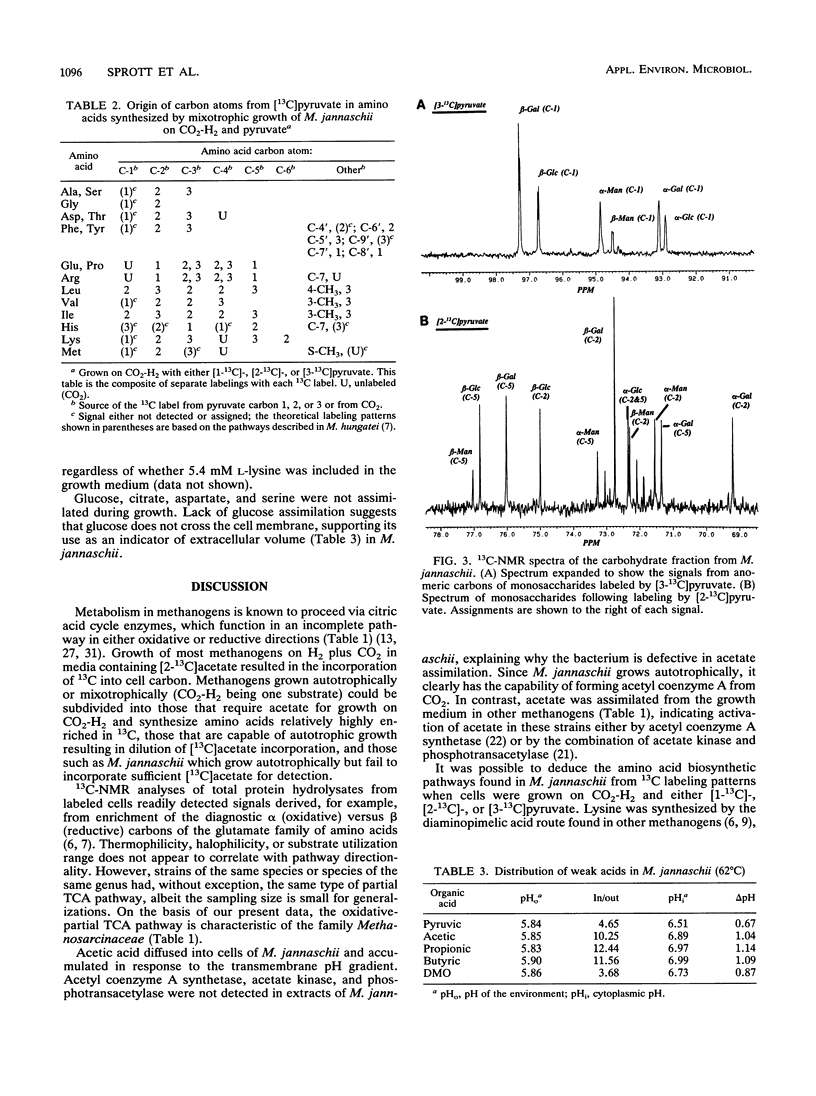
Eleven strains of methanogenic bacteria were divided into two groups on the basis of the directionality (oxidative or reductive) of their citric acid pathways. These pathways were readily identified for most methanogens from the patterns of carbon atom labeling in glutamate, following growth in the presence of [2-13C]acetate. All used noncyclic pathways, but members of the family Methanosarcinaceae were the only methanogens found to use the oxidative direction. Methanococcus jannaschii failed to incorporate carbon from acetate despite transmembrane equilibration comparable to other weak acids. This organism was devoid of detectable activities of the acetate-incorporating enzymes acetyl coenzyme A synthetase, acetate kinase, and phosphotransacetylase. However, incorporation of [1-13C]-, [2-13C]-, or [3-13C]pyruvate during the growth of M. jannaschii was possible and resulted in labeling patterns indicative of a noncyclic citric acid pathway operating in the reductive direction to synthesize amino acids. Carbohydrates were labeled consistent with glucogenesis from pyruvate. Leucine, isoleucine, phenylalanine, lysine, formate, glycerol, and mevalonate were incorporated when supplied to the growth medium. Lysine was preferentially incorporated into the lipid fraction, suggesting a role as a phytanyl chain precursor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Daniels L., Zeikus J. G. One-carbon metabolism in methanogenic bacteria: analysis of short-term fixation products of 14CO2 and 14CH3OH incorporated into whole cells. J Bacteriol. 1978 Oct;136(1):75–84. doi: 10.1128/jb.136.1.75-84.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiel I., Jarrell K. F., Sprott G. D. Amino acid biosynthesis and sodium-dependent transport in Methanococcus voltae, as revealed by 13C NMR. Eur J Biochem. 1985 Jun 3;149(2):437–444. doi: 10.1111/j.1432-1033.1985.tb08944.x. [DOI] [PubMed] [Google Scholar]
- Ekiel I., Smith I. C., Sprott G. D. Biosynthetic pathways in Methanospirillum hungatei as determined by 13C nuclear magnetic resonance. J Bacteriol. 1983 Oct;156(1):316–326. doi: 10.1128/jb.156.1.316-326.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiel I., Sprott G. D., Patel G. B. Acetate and CO2 assimilation by Methanothrix concilii. J Bacteriol. 1985 Jun;162(3):905–908. doi: 10.1128/jb.162.3.905-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiel I., Sprott G. D., Smith I. C. Mevalonic acid is partially synthesized from amino acids in Halobacterium cutirubrum: a 13C nuclear magnetic resonance study. J Bacteriol. 1986 May;166(2):559–564. doi: 10.1128/jb.166.2.559-564.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. N., Tolman C. J., Roberts M. F. Indirect observation by 13C NMR spectroscopy of a novel CO2 fixation pathway in methanogens. Science. 1986 Jan 31;231(4737):488–491. doi: 10.1126/science.3079919. [DOI] [PubMed] [Google Scholar]
- Ferrante G., Richards J. C., Sprott G. D. Structures of polar lipids from the thermophilic, deep-sea archaeobacterium Methanococcus jannaschii. Biochem Cell Biol. 1990 Jan;68(1):274–283. doi: 10.1139/o90-038. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Colvin J. R., Sprott G. D. Spontaneous protoplast formation in Methanobacterium bryantii. J Bacteriol. 1982 Jan;149(1):346–353. doi: 10.1128/jb.149.1.346-353.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Sprott G. D. The transmembrane electrical potential and intracellular pH in methanogenic bacteria. Can J Microbiol. 1981 Jul;27(7):720–728. doi: 10.1139/m81-110. [DOI] [PubMed] [Google Scholar]
- Kenealy W. R., Zeikus J. G. One-carbon metabolism in methanogens: evidence for synthesis of a two-carbon cellular intermediate and unification of catabolism and anabolism in Methanosarcina barkeri. J Bacteriol. 1982 Aug;151(2):932–941. doi: 10.1128/jb.151.2.932-941.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlies G., Fuchs G., Thauer R. K. Acetate thiokinase and the assimilation of acetate in methanobacterium thermoautotrophicum. Arch Microbiol. 1980 Dec;128(2):248–252. doi: 10.1007/BF00406167. [DOI] [PubMed] [Google Scholar]
- Pezacka E., Wood H. G. Role of carbon monoxide dehydrogenase in the autotrophic pathway used by acetogenic bacteria. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6261–6265. doi: 10.1073/pnas.81.20.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. F., Lai M. C., Gunsalus R. P. Biosynthetic pathways of the osmolytes N epsilon-acetyl-beta-lysine, beta-glutamine, and betaine in Methanohalophilus strain FDF1 suggested by nuclear magnetic resonance analyses. J Bacteriol. 1992 Oct;174(20):6688–6693. doi: 10.1128/jb.174.20.6688-6693.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. E., Noll D., Roberts M. F. Free amino acid dynamics in marine methanogens. beta-Amino acids as compatible solutes. J Biol Chem. 1992 Jul 25;267(21):14893–14901. [PubMed] [Google Scholar]
- Schäfer S., Paalme T., Vilu R., Fuchs G. 13C-NMR study of acetate assimilation in Thermoproteus neutrophilus. Eur J Biochem. 1989 Dec 22;186(3):695–700. doi: 10.1111/j.1432-1033.1989.tb15262.x. [DOI] [PubMed] [Google Scholar]
- Shieh J. S., Whitman W. B. Pathway of acetate assimilation in autotrophic and heterotrophic methanococci. J Bacteriol. 1987 Nov;169(11):5327–5329. doi: 10.1128/jb.169.11.5327-5329.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G. D., Ekiel I., Dicaire C. Novel, acid-labile, hydroxydiether lipid cores in methanogenic bacteria. J Biol Chem. 1990 Aug 15;265(23):13735–13740. [PubMed] [Google Scholar]
- Sprott G. D., Shaw K. M., Jarrell K. F. Methanogenesis and the K+ transport system are activated by divalent cations in ammonia-treated cells of Methanospirillum hungatei. J Biol Chem. 1985 Aug 5;260(16):9244–9250. [PubMed] [Google Scholar]
- Sprott G. D., Usher J. R. The electrochemical proton gradient and phenylalanine transport in Escherichia coli irradiated with near-ultraviolet light. Can J Microbiol. 1977 Dec;23(12):1683–1688. doi: 10.1139/m77-242. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate assimilation pathway of Methanosarcina barkeri. J Bacteriol. 1979 Jan;137(1):332–339. doi: 10.1128/jb.137.1.332-339.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Ankwanda E., Wolfe R. S. Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol. 1982 Mar;149(3):852–863. doi: 10.1128/jb.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



