Abstract
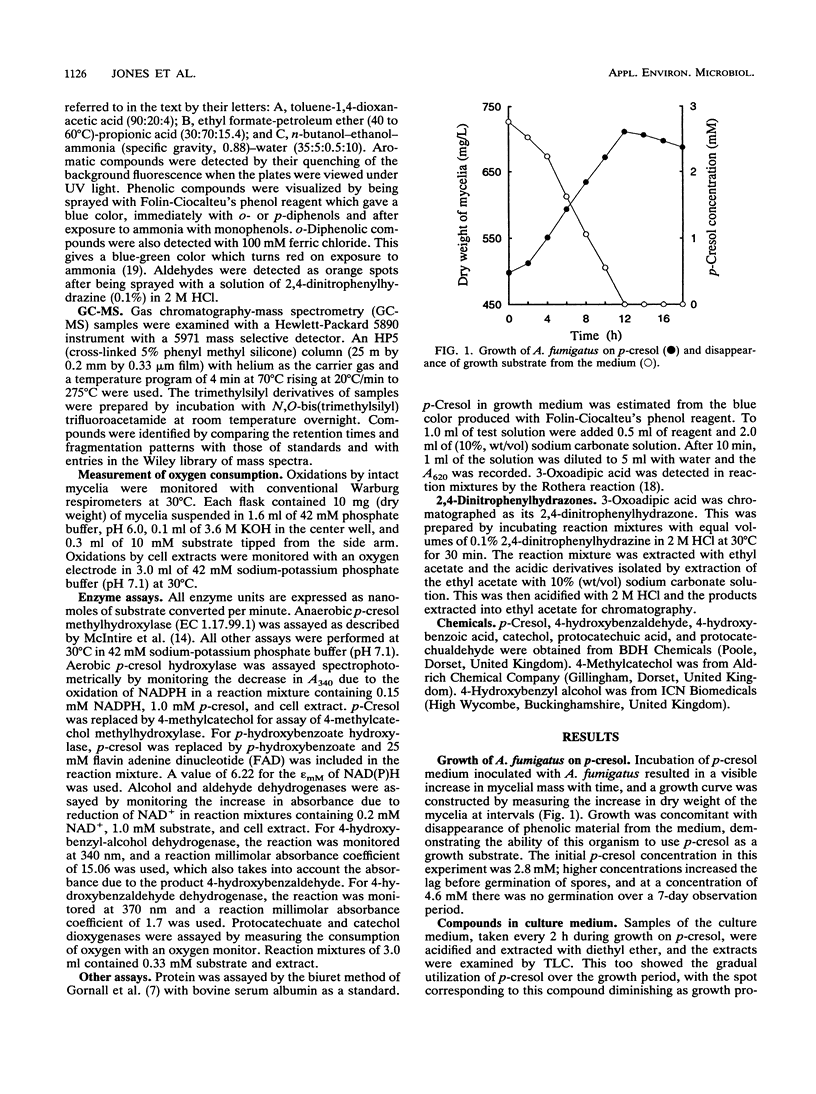
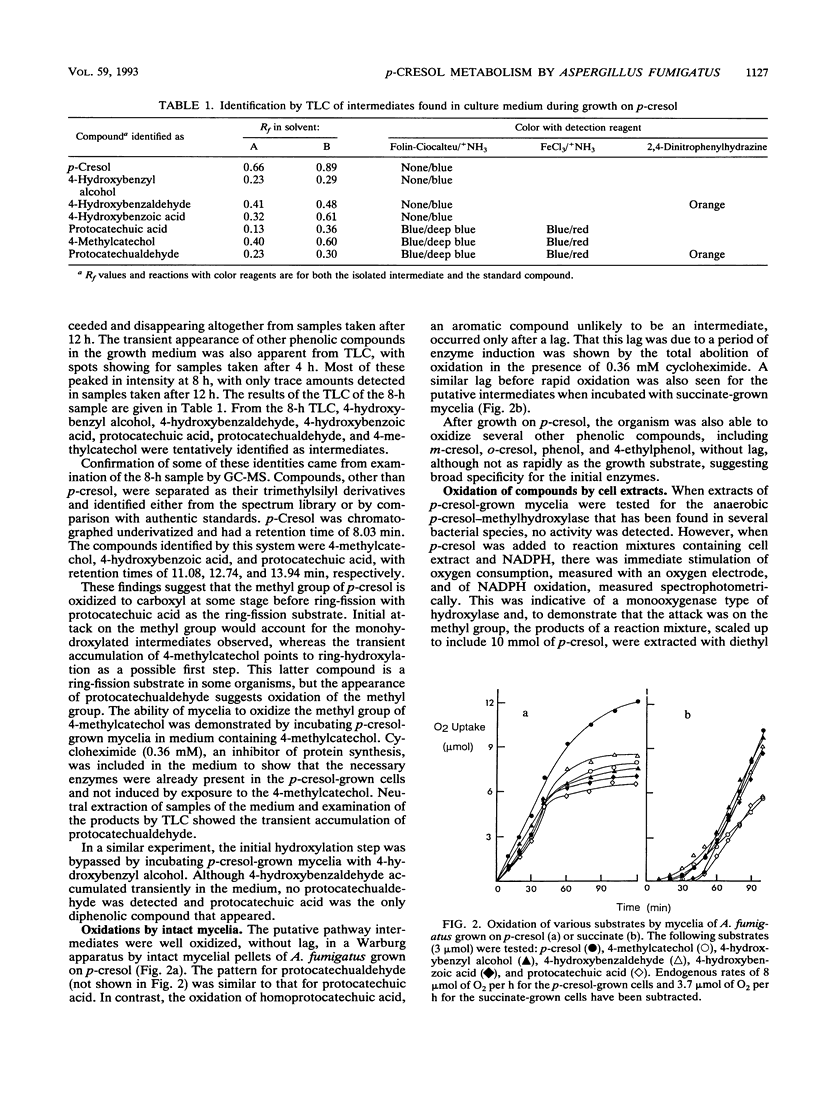
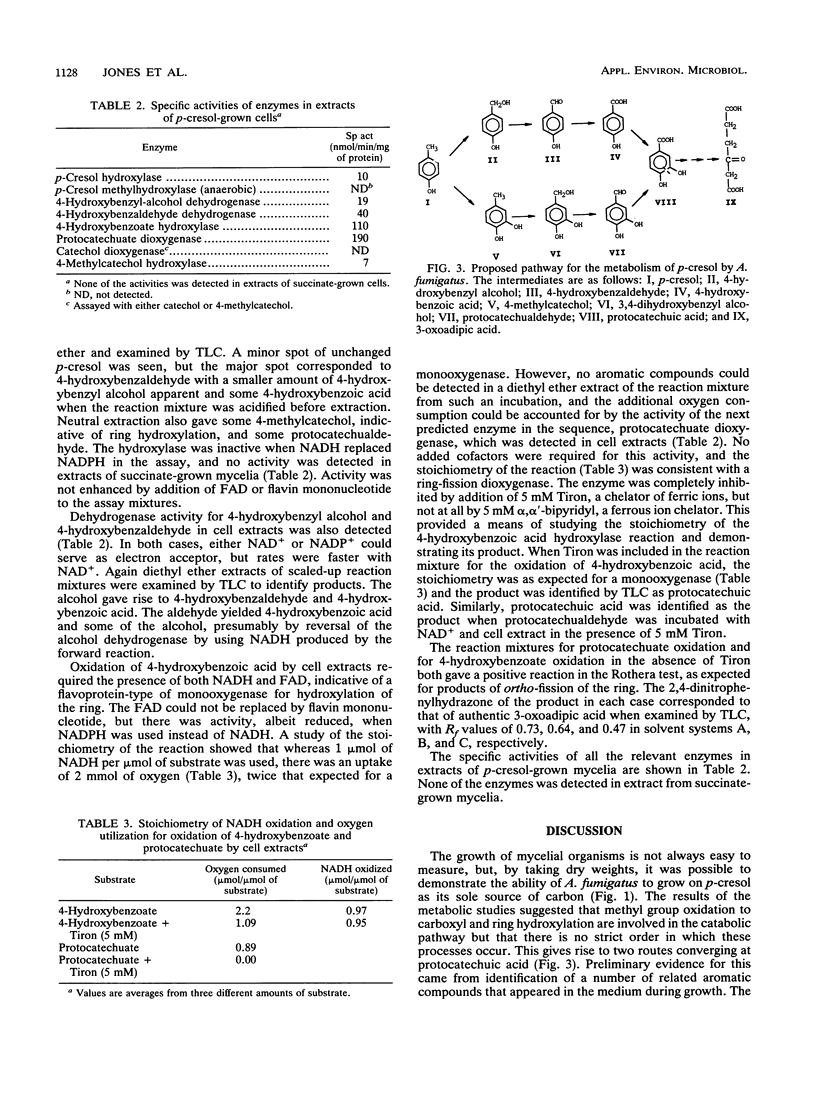
The fungus Aspergillus fumigatus ATCC 28282 was shown to grow on p-cresol as its sole source of carbon and energy. A pathway for metabolism of this compound was proposed. This has protocatechuate as the ring-fission substrate with cleavage and metabolism by an ortho-fission pathway. The protocatechuate was formed by two alternative routes, either by initial attack on the methyl group, which is oxidized to carboxyl, followed by ring-hydroxylation, or by ring-hydroxylation as the first step with subsequent oxidation of 4-methylcatechol to the acid. The pathway was elucidated from several pieces of evidence. A number of compounds, including 4-hydroxybenzyl alcohol, 4-hydroxybenzaldehyde, 4-hydroxybenzoic acid, protocatechuic acid, protocatechualdehyde, and 4-methylcatechol, appeared transiently in the medium during growth on p-cresol. These compounds were oxidized without lag by p-cresol-grown cells but not by succinate-grown cells. Enzyme activities for most of the proposed steps were demonstrated in cell extracts after growth on p-cresol, and the products of these activities were identified. None of the activities were found in succinate-grown cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Dagley S. Catabolism of aromatic acids in Trichosporon cutaneum. J Bacteriol. 1980 Feb;141(2):534–543. doi: 10.1128/jb.141.2.534-543.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert I. D., Young L. Y. Anaerobic oxidation of p-cresol by a denitrifying bacterium. Appl Environ Microbiol. 1986 Nov;52(5):1117–1122. doi: 10.1128/aem.52.5.1117-1122.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ari L., Barker H. A. p-Cresol formation by cell-free extracts of Clostridium difficile. Arch Microbiol. 1985 Dec;143(3):311–312. doi: 10.1007/BF00411256. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., PATEL M. D. Oxidation of p-cresol and related compounds by a Pseudomonas. Biochem J. 1957 Jun;66(2):227–233. doi: 10.1042/bj0660227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Taylor D. G. Pathways for the degradation of m-cresol and p-cresol by Pseudomonas putida. J Bacteriol. 1975 Apr;122(1):1–6. doi: 10.1128/jb.122.1.1-6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K., Stanier R. Y. Crystallization and properties of p-hydroxybenzoate hydroxylase from Pseudomonas putida. J Biol Chem. 1966 May 25;241(10):2453–2460. [PubMed] [Google Scholar]
- Howell L. G., Spector T., Massey V. Purification and properties of p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens. J Biol Chem. 1972 Jul 10;247(13):4340–4350. [PubMed] [Google Scholar]
- Martin A. K. The origin of urinary aromatic compounds excreted by ruminants. 3. The metabolism of phenolic compounds to simple phenols. Br J Nutr. 1982 Nov;48(3):497–507. doi: 10.1079/bjn19820135. [DOI] [PubMed] [Google Scholar]
- McIntire W., Hopper D. J., Singer T. P. p-Cresol methylhydroxylase. Assay and general properties. Biochem J. 1985 Jun 1;228(2):325–335. doi: 10.1042/bj2280325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neujahr H. Y., Gaal A. Phenol hydroxylase from yeast. Purification and properties of the enzyme from Trichosporon cutaneum. Eur J Biochem. 1973 Jun;35(2):386–400. doi: 10.1111/j.1432-1033.1973.tb02851.x. [DOI] [PubMed] [Google Scholar]
- Powlowski J. B., Dagley S. beta-Ketoadipate pathway in Trichosporon cutaneum modified for methyl-substituted metabolites. J Bacteriol. 1985 Sep;163(3):1126–1135. doi: 10.1128/jb.163.3.1126-1135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



