Abstract
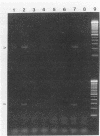
pFL1 is a pUC9 derivative that contains a 572-bp EcoRI insert cloned from plasmid DNA of Xanthomonas campestris pv. citri XC62. The nucleotide sequence of pFL1 was determined, and the sequence information was used to design primers for application of the polymerase chain reaction (PCR) to the detection of X. campestris pv. citri, the causal agent of citrus bacterial canker disease. Seven 18-bp oligonucleotide primers were designed and tested with DNA from X. campestris pv. citri strains and other strains of X. campestris associated with Citrus spp. as templates in the PCR. Four primer pairs directed the amplification of target DNA from X. campestris pv. citri strains but not from strains of X. campestris associated with a different disease, citrus bacterial spot. Primer pair 2-3 directed the specific amplification of target DNA from pathotype A but not other pathotypes of X. campestris pv. citri. A pH 9.0 buffer that contained 1% Triton X-100 and 0.1% gelatin was absolutely required for the successful amplification of the target DNA, which was 61% G+C. Limits of detection after amplification and gel electrophoresis were 25 pg of purified target DNA and about 10 cells when Southern blots were made after gel electrophoresis and probed with biotinylated pFL1. This level of detection represents an increase in sensitivity of about 100-fold over that of dot blotting with the same hybridization probe. PCR products of the expected sizes were amplified from DNA extracted from 7-month-old lesions from which viable bacteria could not be isolated. These products were confirmed to be specific for X. campestris pv. citri by Southern blotting. This PCR-based detection protocol will be a useful addition to current methods of detection of this pathogen, which is currently the target of international quarantine measures.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry T., Colleran G., Glennon M., Dunican L. K., Gannon F. The 16s/23s ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl. 1991 Aug;1(1):51–56. doi: 10.1101/gr.1.1.51. [DOI] [PubMed] [Google Scholar]
- Picard C., Ponsonnet C., Paget E., Nesme X., Simonet P. Detection and enumeration of bacteria in soil by direct DNA extraction and polymerase chain reaction. Appl Environ Microbiol. 1992 Sep;58(9):2717–2722. doi: 10.1128/aem.58.9.2717-2722.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghai-Maroof M. A., Soliman K. M., Jorgensen R. A., Allard R. W. Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci U S A. 1984 Dec;81(24):8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Kapelner S., Sommer S. S. Formamide can dramatically improve the specificity of PCR. Nucleic Acids Res. 1990 Dec 25;18(24):7465–7465. doi: 10.1093/nar/18.24.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]