Abstract
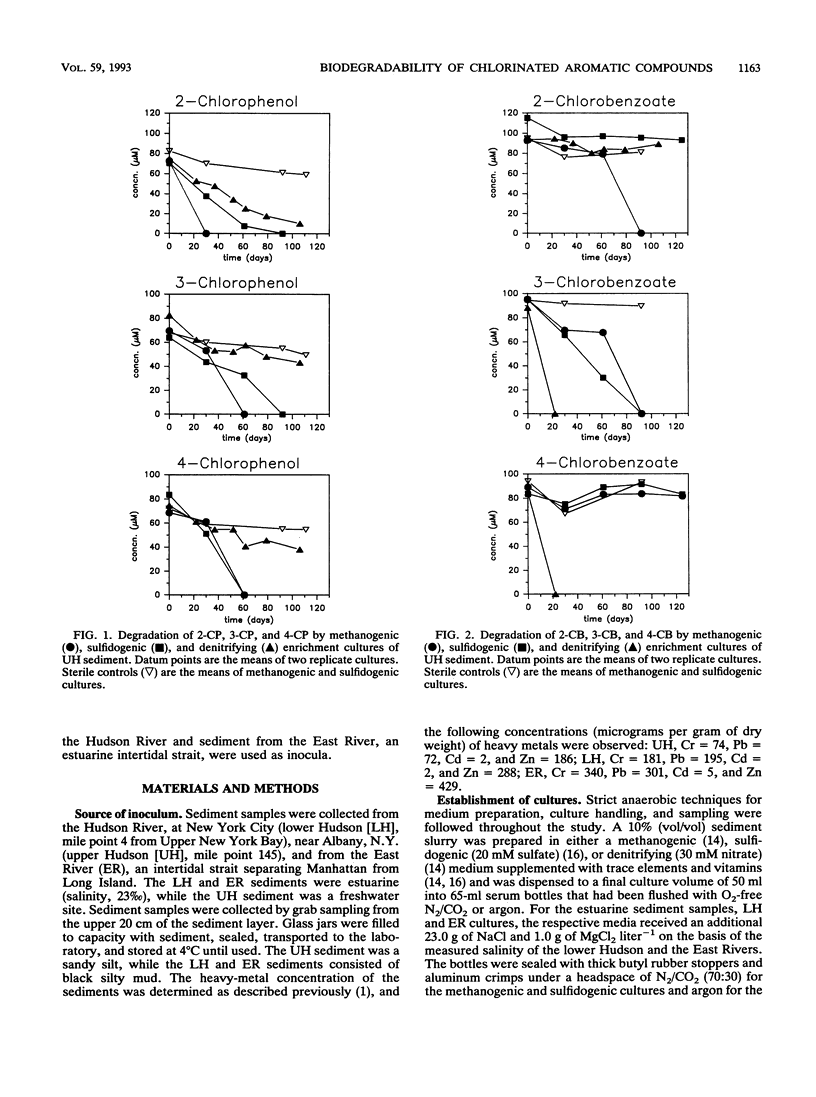
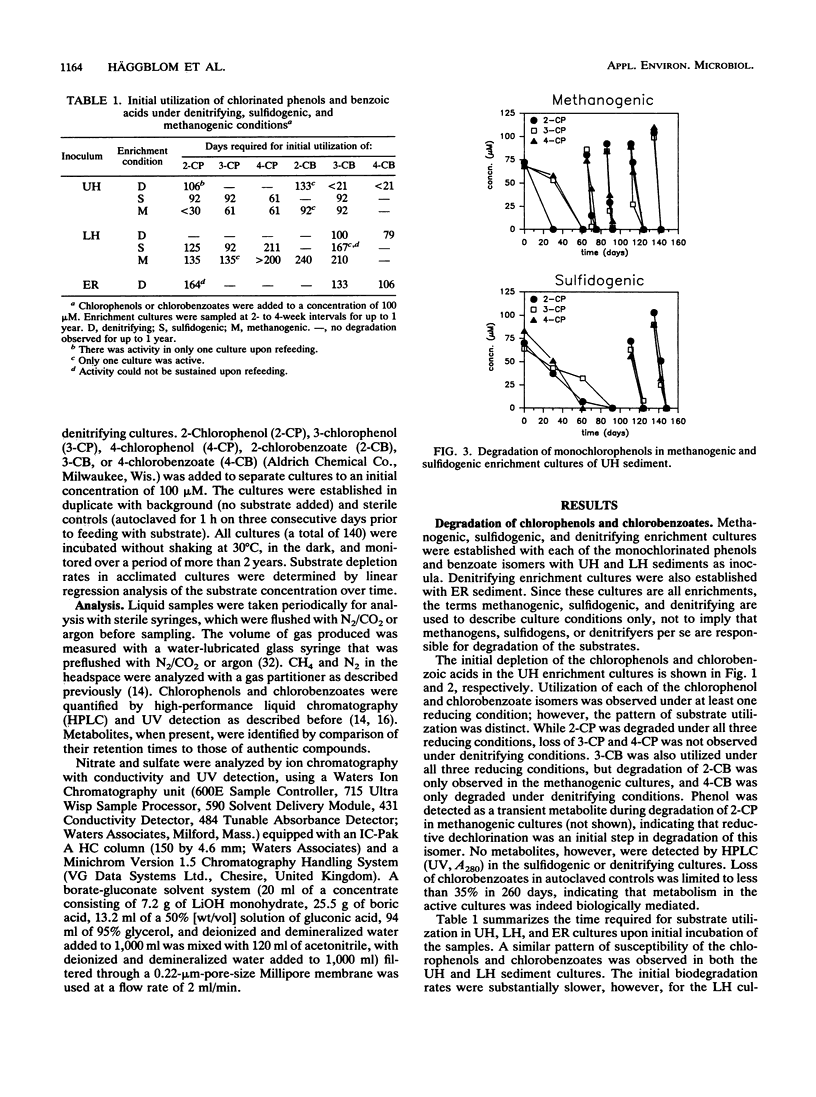
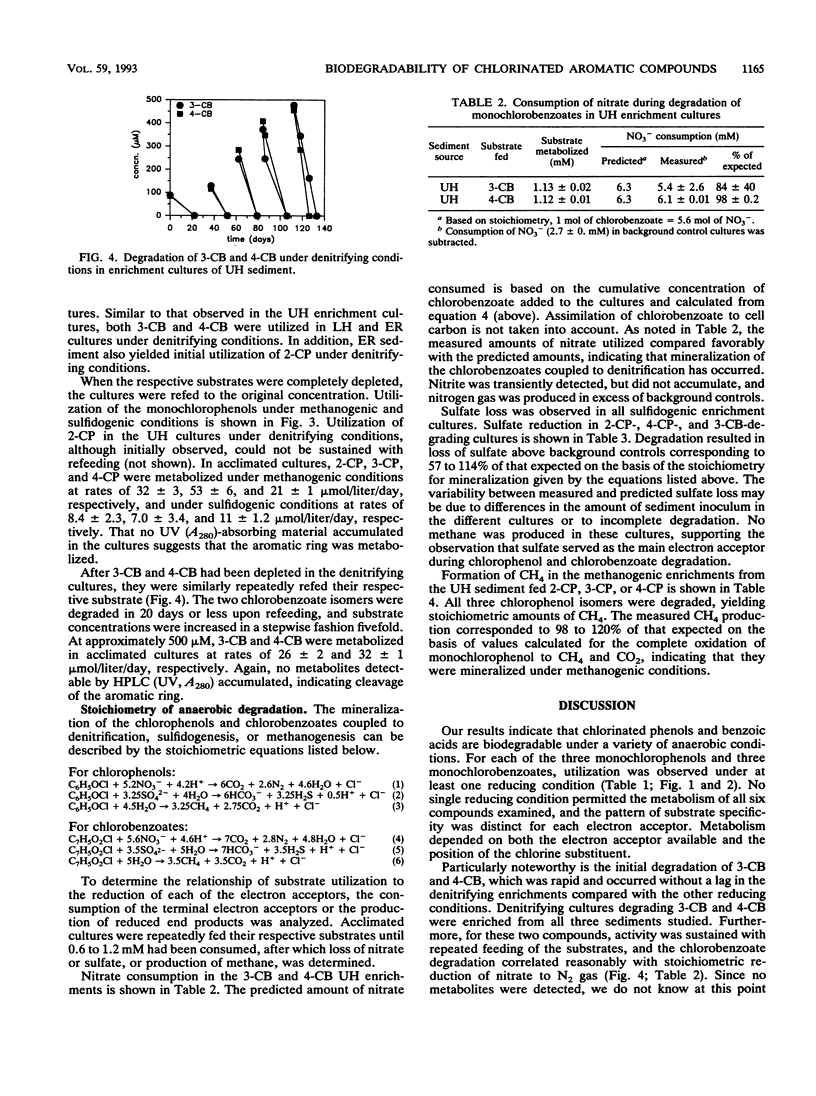
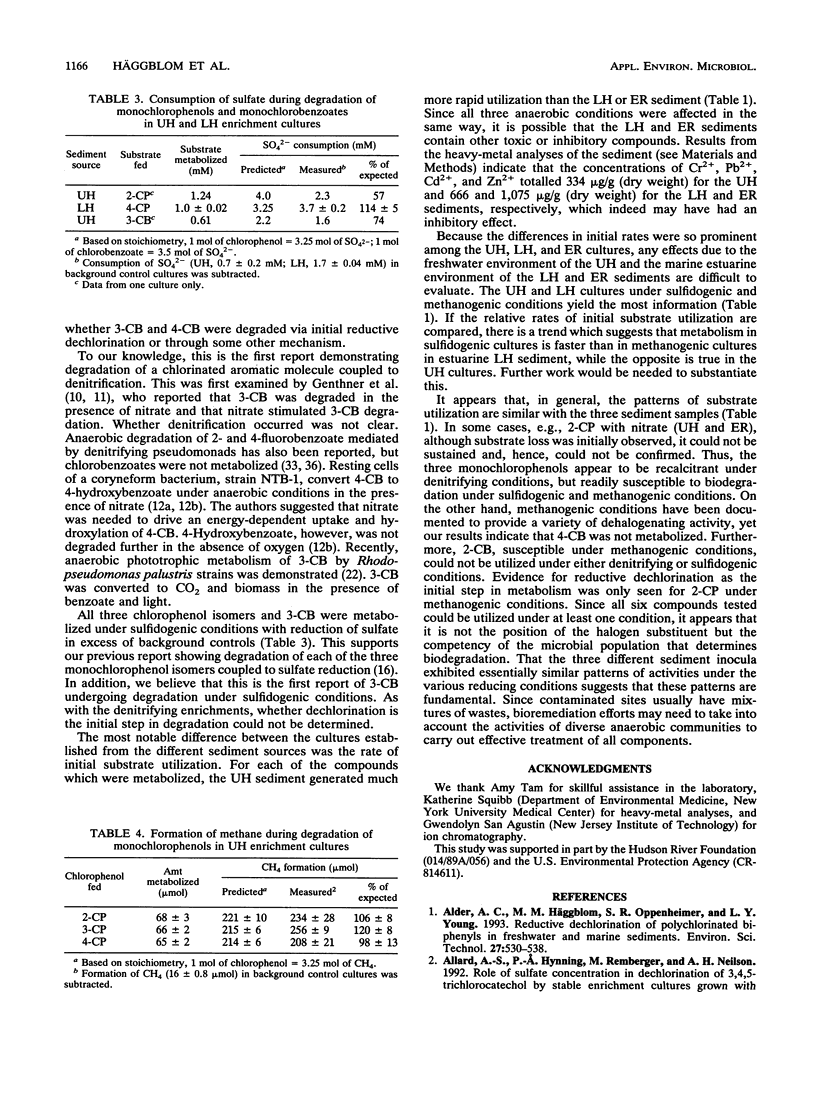
Nitrate, sulfate, and carbonate were used as electron acceptors to examine the anaerobic biodegradability of chlorinated aromatic compounds in estuarine and freshwater sediments. The respective denitrifying, sulfidogenic, and methanogenic enrichment cultures were established on each of the monochlorinated phenol and monochlorinated benzoic acid isomers, using sediment from the upper (freshwater) and lower (estuarine) Hudson River and the East River (estuarine) as source materials. Utilization of each chlorophenol and chlorobenzoate isomer was observed under at least one reducing condition; however, no single reducing condition permitted the metabolism of all six compounds tested. The anaerobic biodegradation of the chlorophenols and chlorobenzoates depended on the electron acceptor available and on the position of the chlorine substituent. In general, similar activities were observed under the different reducing conditions in both the freshwater and estuarine sediments. Under denitrifying conditions, degradation of 3- and 4-chlorobenzoate was accompanied by nitrate loss corresponding reasonably to the stoichiometric values expected for complete oxidation of the chlorobenzoate to CO2. Under sulfidogenic conditions, 3- and 4-chlorobenzoate, but not 2-chlorobenzoate, and all three monochlorophenol isomers were utilized, while under methanogenic conditions all compounds except 4-chlorobenzoate were metabolized. Given that the pattern of activity appears different for these chlorinated compounds under each reducing condition, their biodegradability appears to be more a function of the presence of competent microbial populations than one of inherent molecular structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard A. S., Hynning P. A., Remberger M., Neilson A. H. Role of sulfate concentration in dechlorination of 3,4,5-trichlorocatechol by stable enrichment cultures grown with coumarin and flavanone glycones and aglycones. Appl Environ Microbiol. 1992 Mar;58(3):961–968. doi: 10.1128/aem.58.3.961-968.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd S. A., Shelton D. R. Anaerobic biodegradation of chlorophenols in fresh and acclimated sludge. Appl Environ Microbiol. 1984 Feb;47(2):272–277. doi: 10.1128/aem.47.2.272-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWeerd K. A., Concannon F., Suflita J. M. Relationship between hydrogen consumption, dehalogenation, and the reduction of sulfur oxyanions by Desulfomonile tiedjei. Appl Environ Microbiol. 1991 Jul;57(7):1929–1934. doi: 10.1128/aem.57.7.1929-1934.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Price W. A., Pritchard P. H. Anaerobic Degradation of Chloroaromatic Compounds in Aquatic Sediments under a Variety of Enrichment Conditions. Appl Environ Microbiol. 1989 Jun;55(6):1466–1471. doi: 10.1128/aem.55.6.1466-1471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Price W. A., Pritchard P. H. Characterization of anaerobic dechlorinating consortia derived from aquatic sediments. Appl Environ Microbiol. 1989 Jun;55(6):1472–1476. doi: 10.1128/aem.55.6.1472-1476.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Suflita J. M. Extrapolation of biodegradation results to groundwater aquifers: reductive dehalogenation of aromatic compounds. Appl Environ Microbiol. 1986 Oct;52(4):681–688. doi: 10.1128/aem.52.4.681-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen P. E., Driessen A. J., Konings W. N., de Bont J. A. Energy-dependent uptake of 4-chlorobenzoate in the coryneform bacterium NTB-1. J Bacteriol. 1990 Jan;172(1):419–423. doi: 10.1128/jb.172.1.419-423.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen H. V., Larsen S., Ahring B. K. Influence of a supplemental carbon source on anaerobic dechlorination of pentachlorophenol in granular sludge. Appl Environ Microbiol. 1992 Jan;58(1):365–370. doi: 10.1128/aem.58.1.365-370.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A., Suflita J. M., Tiedje J. M. Reductive dehalogenations of halobenzoates by anaerobic lake sediment microorganisms. Appl Environ Microbiol. 1983 May;45(5):1459–1465. doi: 10.1128/aem.45.5.1459-1465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom M. M. Microbial breakdown of halogenated aromatic pesticides and related compounds. FEMS Microbiol Rev. 1992 Sep;9(1):29–71. doi: 10.1111/j.1574-6968.1992.tb05823.x. [DOI] [PubMed] [Google Scholar]
- Häggblom M. M., Young L. Y. Chlorophenol degradation coupled to sulfate reduction. Appl Environ Microbiol. 1990 Nov;56(11):3255–3260. doi: 10.1128/aem.56.11.3255-3260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal V. S., Wyndham R. C. Anaerobic phototrophic metabolism of 3-chlorobenzoate by Rhodopseudomonas palustris WS17. Appl Environ Microbiol. 1990 Dec;56(12):3871–3873. doi: 10.1128/aem.56.12.3871-3873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M. Dehalogenation in marine sediments containing natural sources of halophenols. Appl Environ Microbiol. 1988 Dec;54(12):3079–3085. doi: 10.1128/aem.54.12.3079-3085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohring G. W., Rogers J. E., Wiegel J. Anaerobic biodegradation of 2,4-dichlorophenol in freshwater lake sediments at different temperatures. Appl Environ Microbiol. 1989 Feb;55(2):348–353. doi: 10.1128/aem.55.2.348-353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohring G. W., Zhang X. M., Wiegel J. Anaerobic dechlorination of 2,4-dichlorophenol in freshwater sediments in the presence of sulfate. Appl Environ Microbiol. 1989 Oct;55(10):2735–2737. doi: 10.1128/aem.55.10.2735-2737.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S., Hendriksen H. V., Ahring B. K. Potential for thermophilic (50 degrees C) anaerobic dechlorination of pentachlorophenol in different ecosystems. Appl Environ Microbiol. 1991 Jul;57(7):2085–2090. doi: 10.1128/aem.57.7.2085-2090.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen T., Aamand H. Anaerobic transformation and toxicity of trichlorophenols in a stable enrichment culture. Appl Environ Microbiol. 1992 Feb;58(2):557–561. doi: 10.1128/aem.58.2.557-561.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen T., Aamand J. Effects of sulfuroxy anions on degradation of pentachlorophenol by a methanogenic enrichment culture. Appl Environ Microbiol. 1991 Sep;57(9):2453–2458. doi: 10.1128/aem.57.9.2453-2458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen T., Licht D. Isolation and characterization of an anaerobic chlorophenol-transforming bacterium. Appl Environ Microbiol. 1992 Sep;58(9):2874–2878. doi: 10.1128/aem.58.9.2874-2878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikesell M. D., Boyd S. A. Complete reductive dechlorination and mineralization of pentachlorophenol by anaerobic microorganisms. Appl Environ Microbiol. 1986 Oct;52(4):861–865. doi: 10.1128/aem.52.4.861-865.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn W. W., Kennedy K. J. Reductive dehalogenation of chlorophenols by Desulfomonile tiedjei DCB-1. Appl Environ Microbiol. 1992 Apr;58(4):1367–1370. doi: 10.1128/aem.58.4.1367-1370.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schennen U., Braun K., Knackmuss H. J. Anaerobic degradation of 2-fluorobenzoate by benzoate-degrading, denitrifying bacteria. J Bacteriol. 1985 Jan;161(1):321–325. doi: 10.1128/jb.161.1.321-325.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suflita J. M., Horowitz A., Shelton D. R., Tiedje J. M. Dehalogenation: a novel pathway for the anaerobic biodegradation of haloaromatic compounds. Science. 1982 Dec 10;218(4577):1115–1117. doi: 10.1126/science.218.4577.1115. [DOI] [PubMed] [Google Scholar]
- Taylor B. F., Hearn W. L., Pincus S. Metabolism of monofluoro- and monochlorobenzoates by a dentrifying bacterium. Arch Microbiol. 1979 Sep;122(3):301–306. doi: 10.1007/BF00411295. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wiegel J. Sequential anaerobic degradation of 2,4-dichlorophenol in freshwater sediments. Appl Environ Microbiol. 1990 Apr;56(4):1119–1127. doi: 10.1128/aem.56.4.1119-1127.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



