Abstract
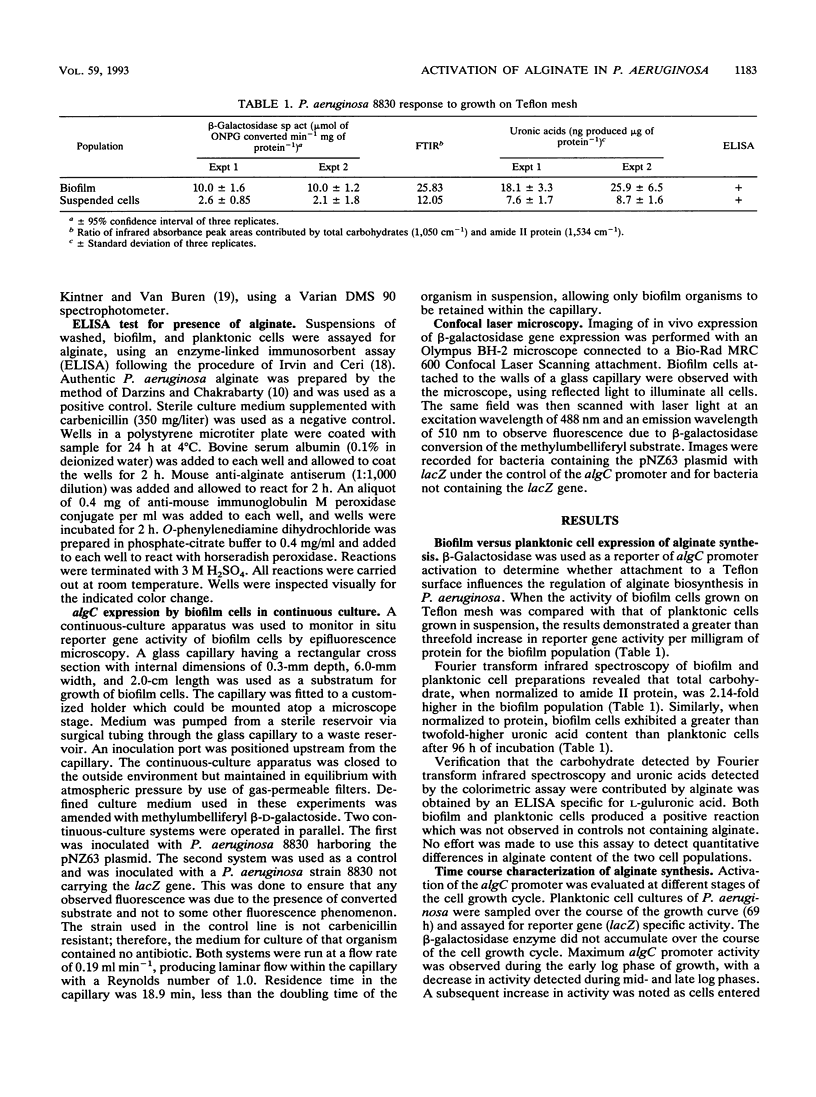
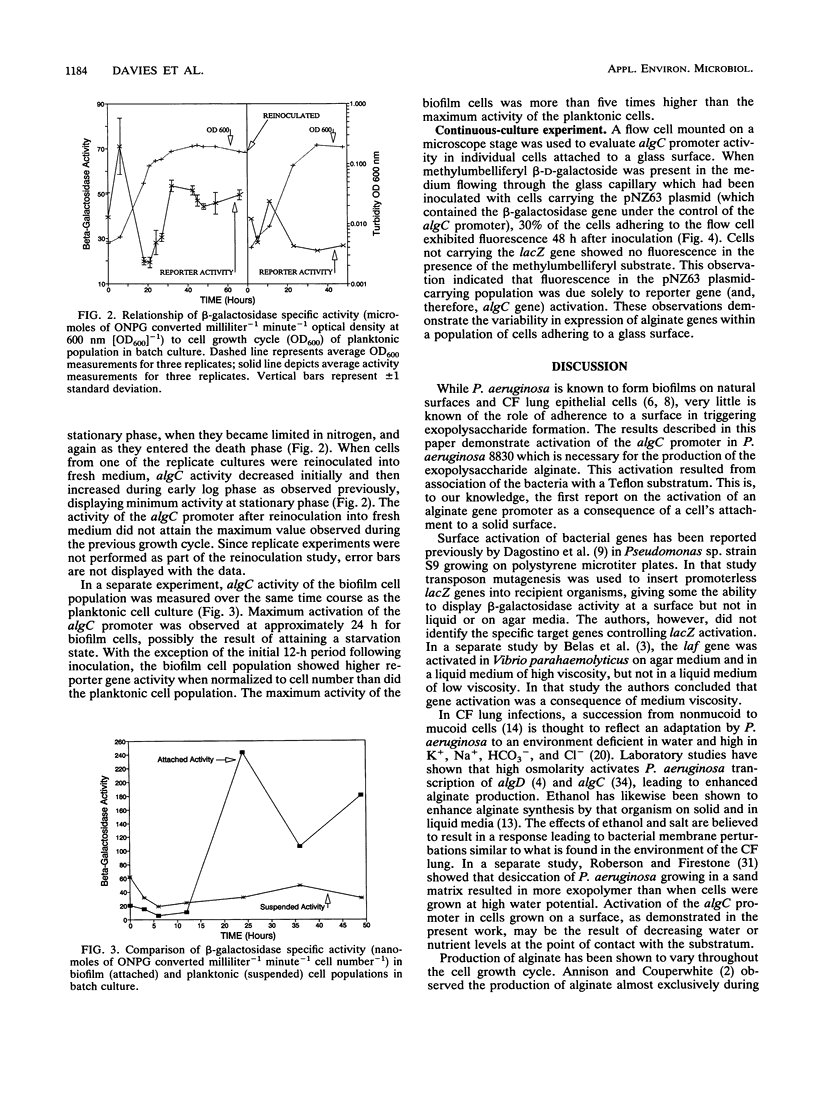
Reporter gene technology was employed to detect the activity of an alginate promoter of Pseudomonas aeruginosa when the organism was grown as a biofilm on a Teflon mesh substratum and as planktonic cells in liquid medium. Alginate biosynthetic activity was determined with a mucoid cell line derived from a cystic fibrosis isolate and containing an alginate algC promoter fused to a lacZ reporter gene. Reporter activity was demonstrated with chromogenic and fluorogenic substrates for beta-galactosidase. Expression of algC was shown to be upregulated in biofilm cells compared with planktonic cells in liquid medium. Gene up-expression correlated with alginate biosynthesis as measured by Fourier transform infrared spectroscopy, uronic acid accumulation, and alginate-specific enzyme-linked immunosorbent assay. The algC promoter was shown to have maximum activity in planktonic cultures during the late lag and early log phases of the cell growth cycle. During a time course experiment, biofilm algC activity exceeded planktonic activity except during the period immediately following inoculation into fresh medium. In continuous-culture experiments, conversion of lacZ substrate was demonstrated microscopically in individual cells by epifluorescence microscopy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annison G., Couperwhite I. Composition of alginate synthesized during the growth cycle of Pseudomonas aeruginosa. Aust J Biol Sci. 1987;40(4):435–441. doi: 10.1071/bi9870435. [DOI] [PubMed] [Google Scholar]
- Belas R., Simon M., Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986 Jul;167(1):210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A., DeVault J. D., Chakrabarty A. M. High osmolarity is a signal for enhanced algD transcription in mucoid and nonmucoid Pseudomonas aeruginosa strains. J Bacteriol. 1989 May;171(5):2312–2317. doi: 10.1128/jb.171.5.2312-2317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVault J. D., Kimbara K., Chakrabarty A. M. Pulmonary dehydration and infection in cystic fibrosis: evidence that ethanol activates alginate gene expression and induction of mucoidy in Pseudomonas aeruginosa. Mol Microbiol. 1990 May;4(5):737–745. doi: 10.1111/j.1365-2958.1990.tb00644.x. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Harris G. S. Pseudomonas aeruginosa and cystic fibrosis: unusual bacterial adaptation and pathogenesis. Microbiol Sci. 1986 Oct;3(10):302–308. [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., Ceri H. Immunochemical examination of the Pseudomonas aeruginosa glycocalyx: a monoclonal antibody which recognizes L-guluronic acid residues of alginic acid. Can J Microbiol. 1985 Mar;31(3):268–275. doi: 10.1139/m85-050. [DOI] [PubMed] [Google Scholar]
- Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., Boucher R. C. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983 Sep 9;221(4615):1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- Knutson C. A., Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968 Sep;24(3):470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- Linker A., Jones R. S. A new polysaccharide resembling alginic acid isolated from pseudomonads. J Biol Chem. 1966 Aug 25;241(16):3845–3851. [PubMed] [Google Scholar]
- May T. B., Shinabarger D., Maharaj R., Kato J., Chu L., DeVault J. D., Roychoudhury S., Zielinski N. A., Berry A., Rothmel R. K. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev. 1991 Apr;4(2):191–206. doi: 10.1128/cmr.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann D., Barnickel G., Bradaczek H., Labischinski H., Giesbrecht P. Infrared spectroscopy, a tool for probing bacterial peptidoglycan. Potentialities of infrared spectroscopy for cell wall analytical studies and rejection of models based on crystalline chitin. Eur J Biochem. 1982 Jul;125(3):505–515. doi: 10.1111/j.1432-1033.1982.tb06711.x. [DOI] [PubMed] [Google Scholar]
- Nickel J. C., Ruseska I., Wright J. B., Costerton J. W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985 Apr;27(4):619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Roberson E. B., Firestone M. K. Relationship between Desiccation and Exopolysaccharide Production in a Soil Pseudomonas sp. Appl Environ Microbiol. 1992 Apr;58(4):1284–1291. doi: 10.1128/aem.58.4.1284-1291.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá-Correia I., Darzins A., Wang S. K., Berry A., Chakrabarty A. M. Alginate biosynthetic enzymes in mucoid and nonmucoid Pseudomonas aeruginosa: overproduction of phosphomannose isomerase, phosphomannomutase, and GDP-mannose pyrophosphorylase by overexpression of the phosphomannose isomerase (pmi) gene. J Bacteriol. 1987 Jul;169(7):3224–3231. doi: 10.1128/jb.169.7.3224-3231.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski N. A., Chakrabarty A. M., Berry A. Characterization and regulation of the Pseudomonas aeruginosa algC gene encoding phosphomannomutase. J Biol Chem. 1991 May 25;266(15):9754–9763. [PubMed] [Google Scholar]
- Zielinski N. A., Maharaj R., Roychoudhury S., Danganan C. E., Hendrickson W., Chakrabarty A. M. Alginate synthesis in Pseudomonas aeruginosa: environmental regulation of the algC promoter. J Bacteriol. 1992 Dec;174(23):7680–7688. doi: 10.1128/jb.174.23.7680-7688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



