Abstract
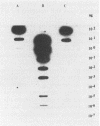
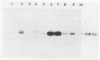
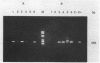
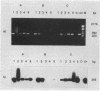
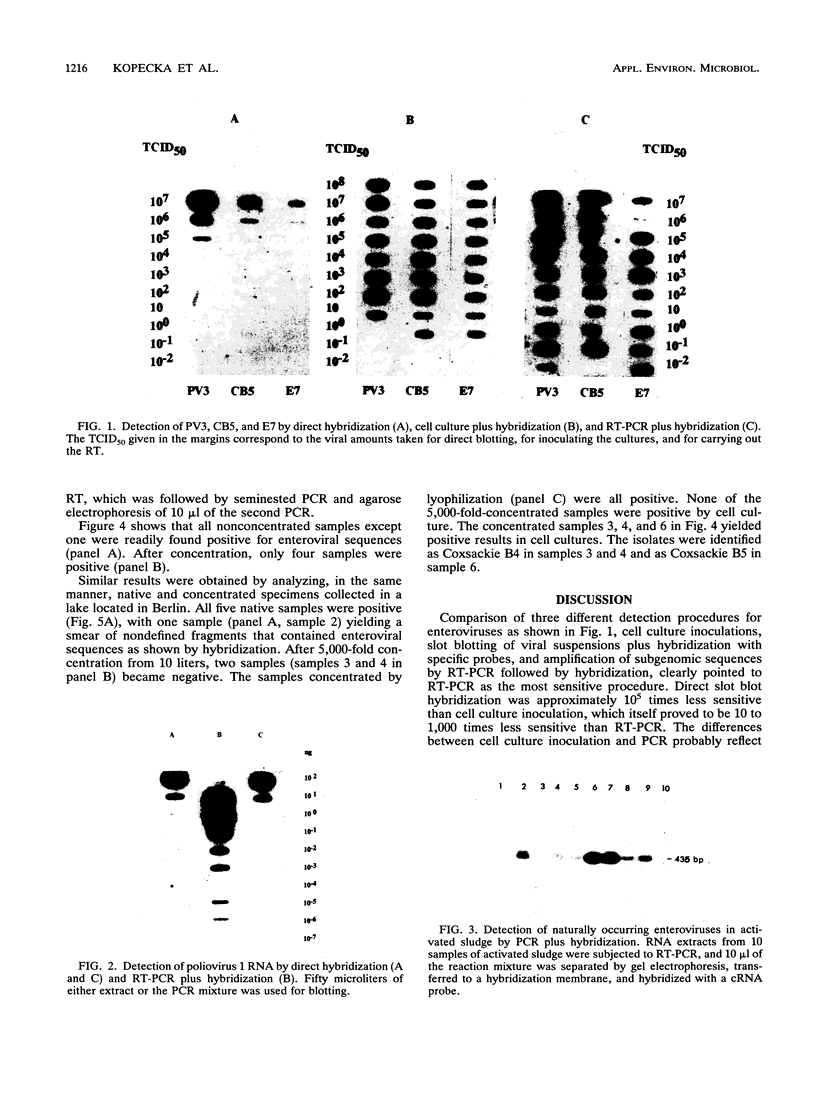
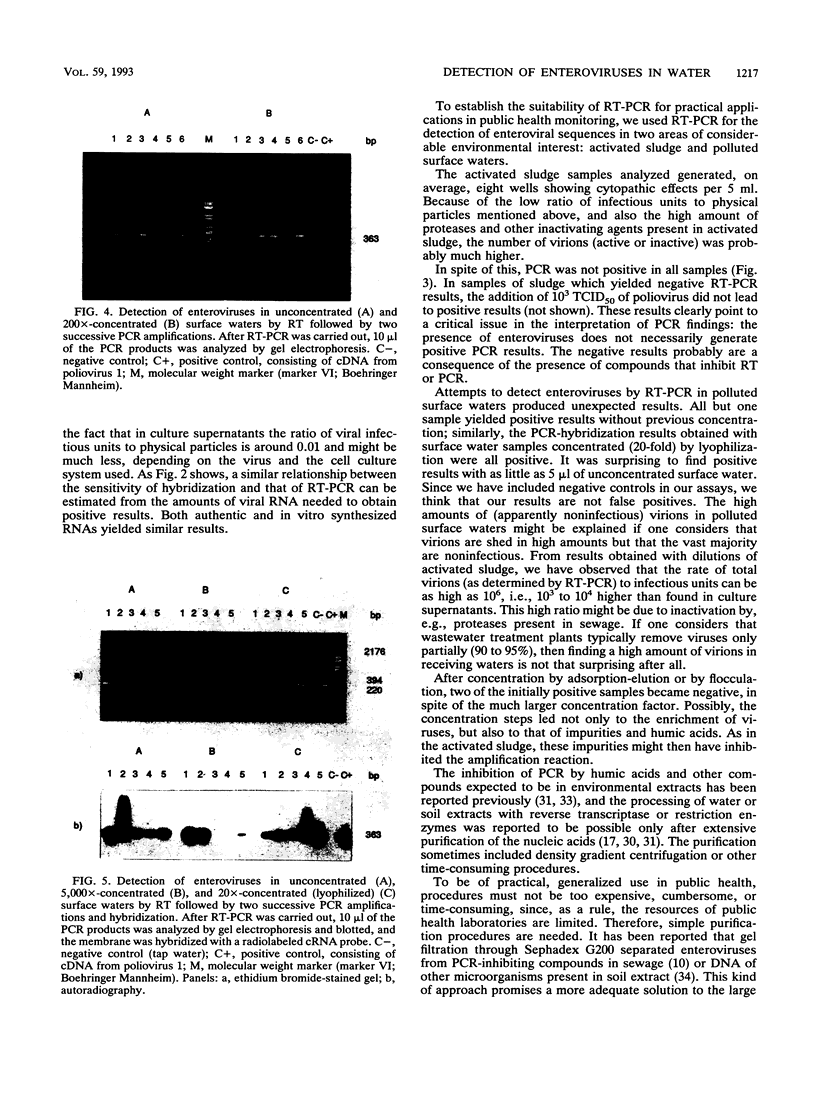
Comparison in virus-seeded mineral water of three detection methods for enteroviruses, direct hybridization, cell culture, and reverse transcription into cDNA followed by polymerase chain reaction and hybridization, showed that the last procedure was 10 to 1,000 times more sensitive than detection by cell culture and 10(5) to 10(7) times more sensitive than direct hybridization. The presence of naturally occurring enteroviruses was also demonstrated in activated sludge and in concentrated and non-concentrated surface water samples by reverse transcription-polymerase chain reaction-hybridization. However, in activated sludge and in concentrated surface waters, enzymatic amplification was sometimes inhibited by contaminants.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bej A. K., Mahbubani M. H., Atlas R. M. Detection of viable Legionella pneumophila in water by polymerase chain reaction and gene probe methods. Appl Environ Microbiol. 1991 Feb;57(2):597–600. doi: 10.1128/aem.57.2.597-600.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bej A. K., Mahbubani M. H., Dicesare J. L., Atlas R. M. Polymerase chain reaction-gene probe detection of microorganisms by using filter-concentrated samples. Appl Environ Microbiol. 1991 Dec;57(12):3529–3534. doi: 10.1128/aem.57.12.3529-3534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauns L. A., Hudson M. C., Oliver J. D. Use of the polymerase chain reaction in detection of culturable and nonculturable Vibrio vulnificus cells. Appl Environ Microbiol. 1991 Sep;57(9):2651–2655. doi: 10.1128/aem.57.9.2651-2655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman N. M., Tracy S., Gauntt C. J., Fortmueller U. Molecular detection and identification of enteroviruses using enzymatic amplification and nucleic acid hybridization. J Clin Microbiol. 1990 May;28(5):843–850. doi: 10.1128/jcm.28.5.843-850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cova L., Kopecka H., Aymard M., Girard M. Use of cRNA probes for the detection of enteroviruses by molecular hybridization. J Med Virol. 1988 Jan;24(1):11–18. doi: 10.1002/jmv.1890240103. [DOI] [PubMed] [Google Scholar]
- Frankel G., Giron J. A., Valmassoi J., Schoolnik G. K. Multi-gene amplification: simultaneous detection of three virulence genes in diarrhoeal stool. Mol Microbiol. 1989 Dec;3(12):1729–1734. doi: 10.1111/j.1365-2958.1989.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Gow J. W., Behan W. M. Amplification and identification of enteroviral sequences in the postviral fatigue syndrome. Br Med Bull. 1991 Oct;47(4):872–885. doi: 10.1093/oxfordjournals.bmb.a072517. [DOI] [PubMed] [Google Scholar]
- Halvorson H. O., Ziegler N. R. Application of Statistics to Problems in Bacteriology: I. A Means of Determining Bacterial Population by the Dilution Method. J Bacteriol. 1933 Feb;25(2):101–121. doi: 10.1128/jb.25.2.101-121.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. E., Keasler S. P., Trucksess M. W., Feng P., Kaysner C. A., Lampel K. A. Polymerase chain reaction identification of Vibrio vulnificus in artificially contaminated oysters. Appl Environ Microbiol. 1991 Mar;57(3):707–711. doi: 10.1128/aem.57.3.707-711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyypiä T., Auvinen P., Maaronen M. Polymerase chain reaction for human picornaviruses. J Gen Virol. 1989 Dec;70(Pt 12):3261–3268. doi: 10.1099/0022-1317-70-12-3261. [DOI] [PubMed] [Google Scholar]
- Iizuka N., Kuge S., Nomoto A. Complete nucleotide sequence of the genome of coxsackievirus B1. Virology. 1987 Jan;156(1):64–73. doi: 10.1016/0042-6822(87)90436-3. [DOI] [PubMed] [Google Scholar]
- Jenkins O., Booth J. D., Minor P. D., Almond J. W. The complete nucleotide sequence of coxsackievirus B4 and its comparison to other members of the Picornaviridae. J Gen Virol. 1987 Jul;68(Pt 7):1835–1848. doi: 10.1099/0022-1317-68-7-1835. [DOI] [PubMed] [Google Scholar]
- Jin O., Sole M. J., Butany J. W., Chia W. K., McLaughlin P. R., Liu P., Liew C. C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation. 1990 Jul;82(1):8–16. doi: 10.1161/01.cir.82.1.8. [DOI] [PubMed] [Google Scholar]
- Kopecka H., Prévot J., Girard M., Fuchs F., Aymard M. Intérêt des sondes ARNc (ribosondes) synthétisés in vitro dans la détection des entérovirus par hybridation moléculaire. Ann Inst Pasteur Virol. 1988 Apr-Jun;139(2):217–225. doi: 10.1016/s0769-2617(88)80019-4. [DOI] [PubMed] [Google Scholar]
- Lindberg A. M., Stålhandske P. O., Pettersson U. Genome of coxsackievirus B3. Virology. 1987 Jan;156(1):50–63. doi: 10.1016/0042-6822(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Mahbubani M. H., Bej A. K., Perlin M., Schaefer F. W., 3rd, Jakubowski W., Atlas R. M. Detection of Giardia cysts by using the polymerase chain reaction and distinguishing live from dead cysts. Appl Environ Microbiol. 1991 Dec;57(12):3456–3461. doi: 10.1128/aem.57.12.3456-3461.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive D. M., Al-Mufti S., Al-Mulla W., Khan M. A., Pasca A., Stanway G., Al-Nakib W. Detection and differentiation of picornaviruses in clinical samples following genomic amplification. J Gen Virol. 1990 Sep;71(Pt 9):2141–2147. doi: 10.1099/0022-1317-71-9-2141. [DOI] [PubMed] [Google Scholar]
- Reyes G. R., Purdy M. A., Kim J. P., Luk K. C., Young L. M., Fry K. E., Bradley D. W. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990 Mar 16;247(4948):1335–1339. doi: 10.1126/science.2107574. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A. Enzymatic RNA amplification of the enteroviruses. J Clin Microbiol. 1990 Mar;28(3):438–442. doi: 10.1128/jcm.28.3.438-442.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart H. A., Levin M. J., Villarreal L. P. Use of subgenomic poliovirus DNA hybridization probes to detect the major subgroups of enteroviruses. J Clin Microbiol. 1984 Dec;20(6):1105–1108. doi: 10.1128/jcm.20.6.1105-1108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. Polymerase chain reaction: applications in environmental microbiology. Annu Rev Microbiol. 1991;45:137–161. doi: 10.1146/annurev.mi.45.100191.001033. [DOI] [PubMed] [Google Scholar]
- Steffan R. J., Goksøyr J., Bej A. K., Atlas R. M. Recovery of DNA from soils and sediments. Appl Environ Microbiol. 1988 Dec;54(12):2908–2915. doi: 10.1128/aem.54.12.2908-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H., Kohara M., Kataoka Y., Suganuma T., Omata T., Imura N., Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol. 1984 Apr 25;174(4):561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Detection of low numbers of bacterial cells in soils and sediments by polymerase chain reaction. Appl Environ Microbiol. 1992 Feb;58(2):754–757. doi: 10.1128/aem.58.2.754-757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Rapid method for separation of bacterial DNA from humic substances in sediments for polymerase chain reaction. Appl Environ Microbiol. 1992 Jul;58(7):2292–2295. doi: 10.1128/aem.58.7.2292-2295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter R., Rüdiger S. Ein Zweistufenverfahren Zur Virusanreicherung Aus Lösungen Mit gerringem Virustiter (Z. B. Trinkwasser). J Hyg Epidemiol Microbiol Immunol. 1981;25(1):71–81. [PubMed] [Google Scholar]
- Wernars K., Delfgou E., Soentoro P. S., Notermans S. Successful approach for detection of low numbers of enterotoxigenic Escherichia coli in minced meat by using the polymerase chain reaction. Appl Environ Microbiol. 1991 Jul;57(7):1914–1919. doi: 10.1128/aem.57.7.1914-1919.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. C., Purcell R. H., Sreenivasan M. A., Prasad S. R., Pavri K. M. Epidemic and endemic hepatitis in India: evidence for a non-A, non-B hepatitis virus aetiology. Lancet. 1980 Oct 25;2(8200):876–879. doi: 10.1016/s0140-6736(80)92045-0. [DOI] [PubMed] [Google Scholar]
- Zoll G. J., Melchers W. J., Kopecka H., Jambroes G., van der Poel H. J., Galama J. M. General primer-mediated polymerase chain reaction for detection of enteroviruses: application for diagnostic routine and persistent infections. J Clin Microbiol. 1992 Jan;30(1):160–165. doi: 10.1128/jcm.30.1.160-165.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]