Abstract
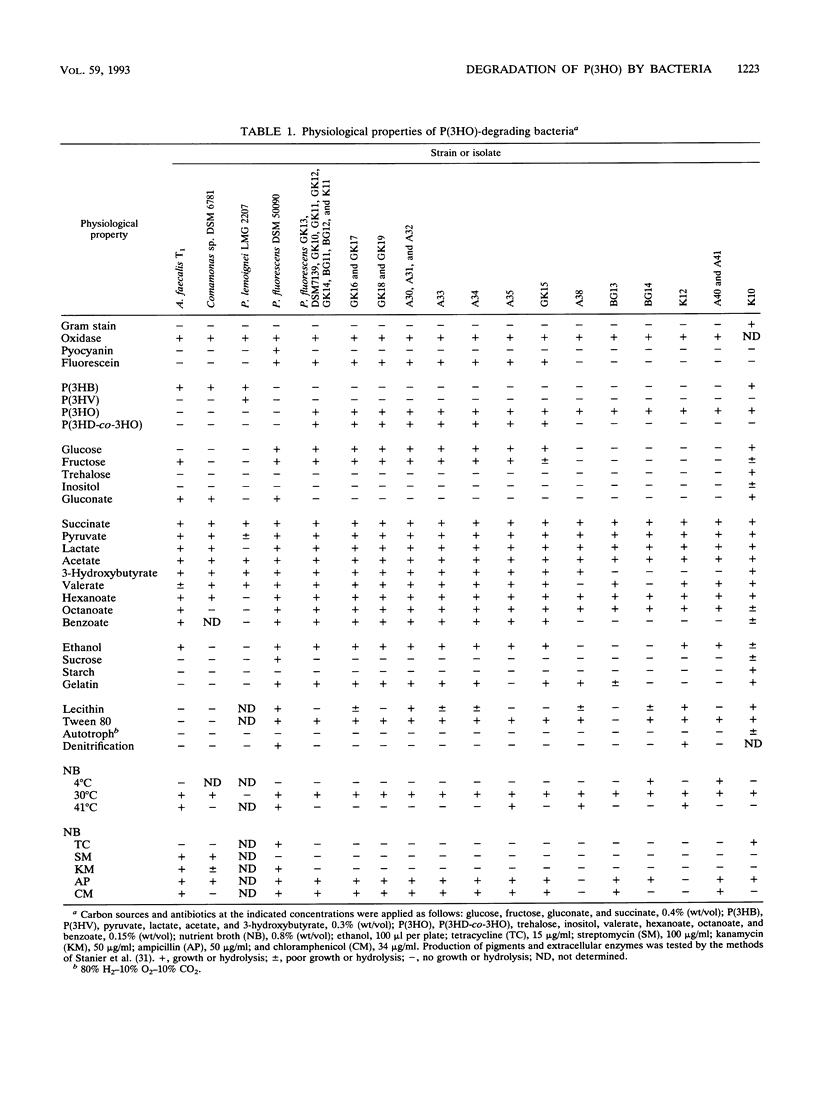
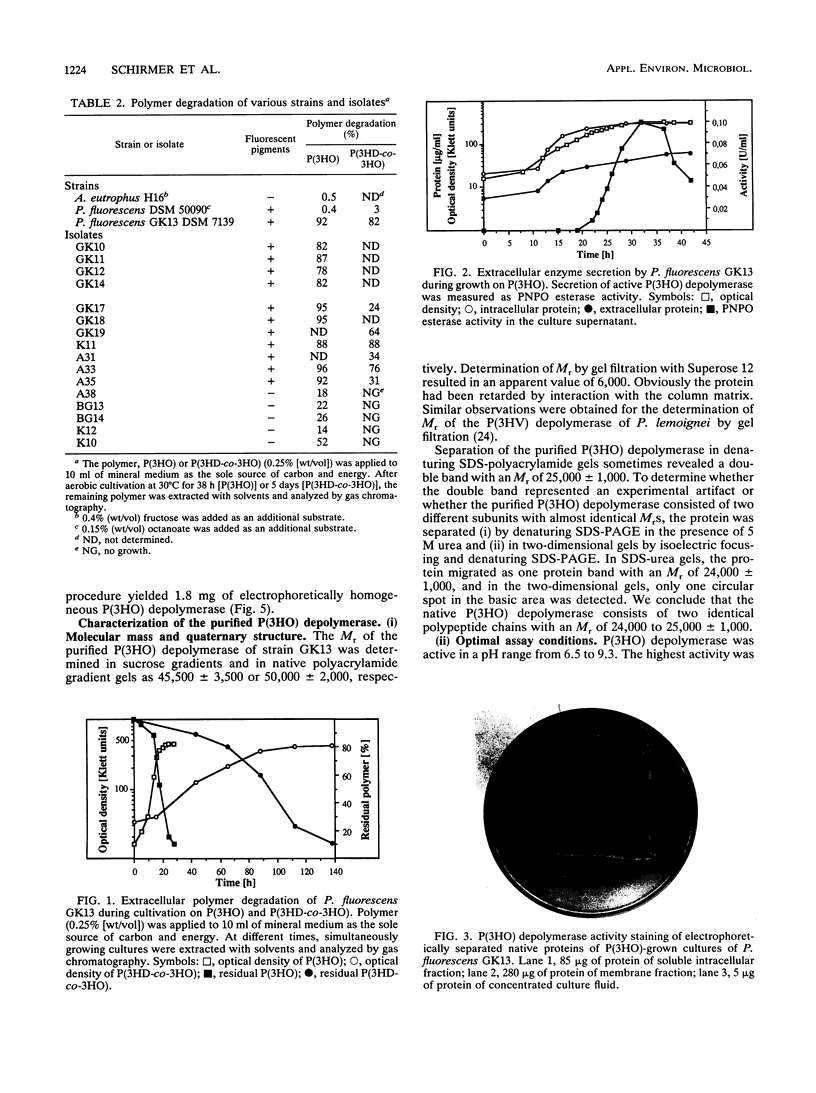
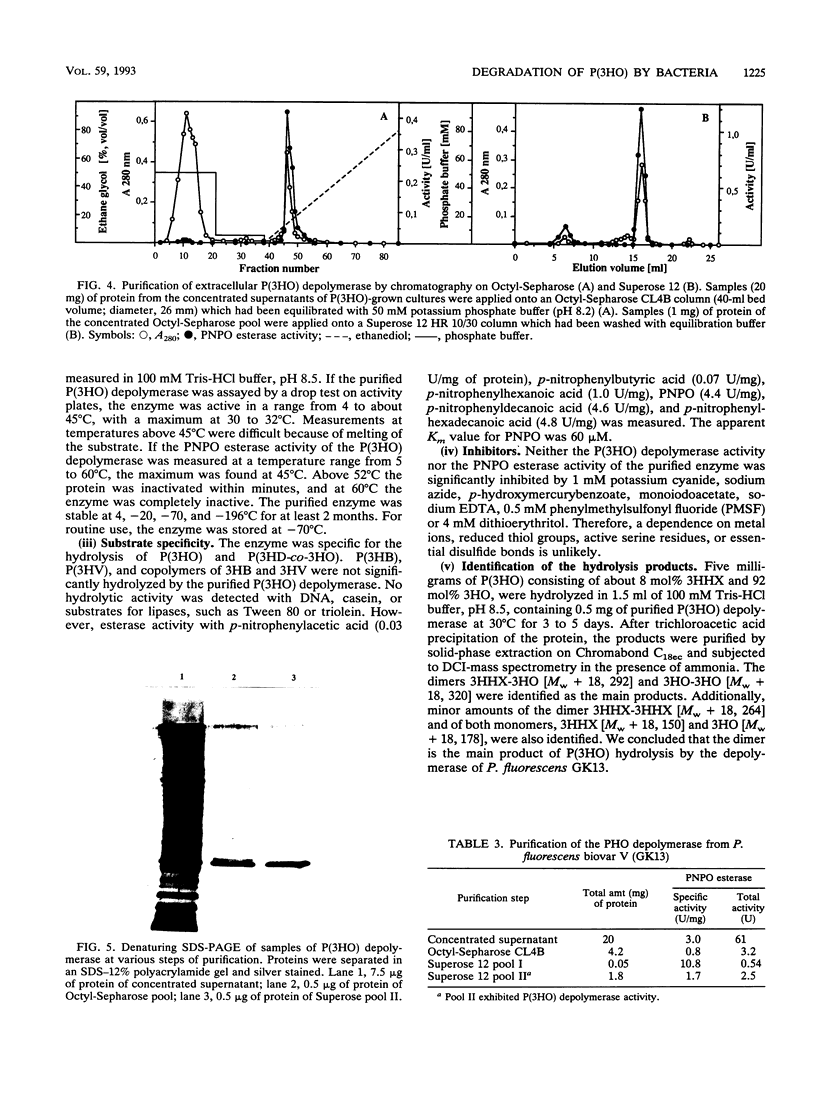
Twenty-five gram-negative bacteria and one gram-positive bacterium capable of growing on poly(3-hydroxyoctanoic acid) [P(3HO)] as the sole source of carbon and energy were isolated from various soils, lake water, and activated sludge. Most of the isolates degraded only P(3HO) and copolymers of medium-chain-length (MCL) hydroxyalkanoic acids (HA). Except for the gram-positive strain, which was able to hydrolyze P(3HO) and poly(3-hydroxybutyric acid) [P(3HB)], no isolate was able to degrade polymers of short-chain-length HA, such as P(3HB) or poly(3-hydroxyvalerate) [P(3HV)]. All strains utilized a large variety of monomeric substrates for growth. All gram-negative strains, but not the gram-positive strain, accumulated poly(hydroxyalkanoic acids) (PHA), consisting of MCL HA, if they were cultivated under accumulation conditions. One strain, which was identified as Pseudomonas fluorescens GK13 (biovar V), was selected and the extracellular P(3HO) depolymerase of this strain was purified from the culture medium of P(3HO)-grown cells by chromatography with Octyl-Sepharose CL4B and by gel filtration with Superose 12. The relative molecular weights of the native and sodium dodecyl sulfate-treated enzymes were 48,000 and 25,000, respectively. The purified enzyme hydrolyzed P(3HO), copolymers of MCL HA, and para-nitrophenyl esters of fatty acids. P(3HB), P(3HV), and characteristic substrates for lipases, such as Tween 80 or triolein, were not hydrolyzed. The P(3HO) depolymerase of P. fluorescens GK13 was insensitive to phenylmethylsulfonyl fluoride and dithioerythritol, unlike other PHA depolymerases. The dimeric ester of 3-hydroxyoctanoic acid was identified as the main product of enzymatic hydrolysis of P(3HO).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Dawes E. A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990 Dec;54(4):450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl H., Gross R. A., Lenz R. W., Fuller R. C. Pseudomonas oleovorans as a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl Environ Microbiol. 1988 Aug;54(8):1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budwill K., Fedorak P. M., Page W. J. Methanogenic degradation of poly(3-hydroxyalkanoates). Appl Environ Microbiol. 1992 Apr;58(4):1398–1401. doi: 10.1128/aem.58.4.1398-1401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafield F. P., Doudoroff M., Palleroni N. J., Lusty C. J., Contopoulos R. Decomposition of poly-beta-hydroxybutyrate by pseudomonads. J Bacteriol. 1965 Nov;90(5):1455–1466. doi: 10.1128/jb.90.5.1455-1466.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORSYTH W. G., HAYWARD A. C., ROBERTS J. B. Occurrence of poly-beta-hydroxybutyric acid in aerobic gram-negative bacteria. Nature. 1958 Sep 20;182(4638):800–801. doi: 10.1038/182800a0. [DOI] [PubMed] [Google Scholar]
- Haywood G. W., Anderson A. J., Ewing D. F., Dawes E. A. Accumulation of a Polyhydroxyalkanoate Containing Primarily 3-Hydroxydecanoate from Simple Carbohydrate Substrates by Pseudomonas sp. Strain NCIMB 40135. Appl Environ Microbiol. 1990 Nov;56(11):3354–3359. doi: 10.1128/aem.56.11.3354-3359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood G. W., Anderson A. J., Williams D. R., Dawes E. A., Ewing D. F. Accumulation of a poly(hydroxyalkanoate) copolymer containing primarily 3-hydroxyvalerate from simple carbohydrate substrates by Rhodococcus sp. NCIMB 40126. Int J Biol Macromol. 1991 Apr;13(2):83–88. doi: 10.1016/0141-8130(91)90053-w. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lageveen R. G., Huisman G. W., Preusting H., Ketelaar P., Eggink G., Witholt B. Formation of Polyesters by Pseudomonas oleovorans: Effect of Substrates on Formation and Composition of Poly-(R)-3-Hydroxyalkanoates and Poly-(R)-3-Hydroxyalkenoates. Appl Environ Microbiol. 1988 Dec;54(12):2924–2932. doi: 10.1128/aem.54.12.2924-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory S. Determinants of extracellular protein secretion in gram-negative bacteria. J Bacteriol. 1992 Jun;174(11):3423–3428. doi: 10.1128/jb.174.11.3423-3428.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusty C. J., Doudoroff M. Poly-beta-hydroxybutyrate depolymerases of Pseudomonas lemoignei. Proc Natl Acad Sci U S A. 1966 Sep;56(3):960–965. doi: 10.1073/pnas.56.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Saito T., Fukui T., Shirakura Y., Tomita K. Purification and properties of extracellular poly(3-hydroxybutyrate) depolymerases from Pseudomonas lemoignei. Biochim Biophys Acta. 1985 Jan 21;827(1):63–72. doi: 10.1016/0167-4838(85)90101-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- SCHLEGEL H. G., GOTTSCHALK G., VON BARTHA R. Formation and utilization of poly-beta-hydroxybutyric acid by Knallgas bacteria (Hydrogenomonas). Nature. 1961 Jul 29;191:463–465. doi: 10.1038/191463a0. [DOI] [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Shirakura Y., Fukui T., Saito T., Okamoto Y., Narikawa T., Koide K., Tomita K., Takemasa T., Masamune S. Degradation of poly(3-hydroxybutyrate) by poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis T1. Biochim Biophys Acta. 1986 Jan 15;880(1):46–53. doi: 10.1016/0304-4165(86)90118-2. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Stegemann H., Francksen H., Macko V. Potato proteins: genetic and physiological changes, evaluated by one- and two-dimensional PAA-gel-techniques. Z Naturforsch C. 1973 Nov-Dec;28(11):722–732. doi: 10.1515/znc-1973-11-1213. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Merrick J. M. Extracellular enzyme secretion by Pseudomonas lemoignei. J Bacteriol. 1974 Jul;119(1):152–161. doi: 10.1128/jb.119.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanio T., Fukui T., Shirakura Y., Saito T., Tomita K., Kaiho T., Masamune S. An extracellular poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis. Eur J Biochem. 1982 May;124(1):71–77. doi: 10.1111/j.1432-1033.1982.tb05907.x. [DOI] [PubMed] [Google Scholar]
- Timm A., Steinbüchel A. Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent pseudomonads. Appl Environ Microbiol. 1990 Nov;56(11):3360–3367. doi: 10.1128/aem.56.11.3360-3367.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet M. J., Eggink G., Witholt B., Kingma J., Wynberg H. Characterization of intracellular inclusions formed by Pseudomonas oleovorans during growth on octane. J Bacteriol. 1983 May;154(2):870–878. doi: 10.1128/jb.154.2.870-878.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]