Abstract
Background
The benefits of highly active antiretroviral therapy (HAART) for the treatment of HIV infection are well documented, but concerns regarding access and adherence to HAART are growing. We evaluated virological responses to HAART among HIV-1 infected patients who were injection drug users (IDUs) in a population-based setting where HIV/AIDS care is delivered free of charge.
Methods
We evaluated previously untreated HIV-1 infected men and women who initiated HAART between Aug. 1, 1996, and July 31, 2000, and who were followed until Mar. 31, 2002, in a province-wide HIV treatment program. We used Kaplan–Meier methods and Cox proportional hazards regression in our evaluation of time to suppression (i.e., less than 500 copies/mL) and rebound (i.e., 500 copies/mL or more) of plasma HIV-1 RNA, with patients stratified according to whether or not they had a history of injection drug use.
Results
Overall, 1422 patients initiated HAART during the study period, of whom 359 (25.2%) were IDUs. In Kaplan–Meier analyses, the cumulative suppression rate at 12 months after initiation of HAART was 70.8% for non-IDUs and 51.4% for IDUs (p < 0.001) (these values include people who achieved suppression before 12 months but who might not have been followed for the full 12-month period). Among patients who achieved suppression of plasma HIV-1 RNA, the cumulative rebound rate at 12 months after initial suppression was 23.8% for non-IDUs and 34.7% for IDUs (p < 0.001). However, after adjustment for adherence and other covariates, the rates of HIV-1 RNA suppression (adjusted relative hazard 0.9, 95% confidence interval [CI] 0.7–1.0) and HIV-1 RNA rebound (adjusted relative hazard 1.3, 95% CI 1.0–1.6) were similar between non-IDUs and IDUs. Differences between non-IDUs and IDUs were even less pronounced in subanalyses that considered only therapy-adherent patients (p > 0.1).
Interpretation
Non-IDUs and IDUs had similar rates of HIV-1 RNA suppression and rebound after the initiation of HAART, once lower levels of adherence were taken into account. Nevertheless, the lower virological response rates among IDUs suggest that, unless interventions are undertaken to improve adherence, these patients may experience elevated rates of disease progression and use of medical services in our setting.
The benefits of triple-drug highly active antiretroviral therapy (HAART) in the management of HIV disease are well established. Through the suppression of plasma HIV-1 RNA, HAART has been shown to improve CD4 cell counts and in turn to decrease morbidity and mortality rates among HIV–infected patients.1,2 As a result, HAART has become the standard of care for HIV-infected patients.3
In North America, these findings have emerged amid growing concerns about inequitable access to antiretroviral therapy and lower levels of adherence among those who were infected during later stages of the HIV epidemic, particularly illicit injection drug users (IDUs).4,5,6 In British Columbia, the majority of HIV infections among IDUs did not occur until 1996,7 and hence the bulk of HIV-infected IDUs will only be starting to require HIV treatment over the next several years.3,8 An earlier analysis from our setting suggested that IDUs had rates of HIV-1 RNA suppression similar to those of non-IDUs when adherence was taken into account.9 However, this earlier work did not report rates of failure to achieve HIV-1 RNA suppression among IDUs, nor did it report rates of HIV-1 RNA rebound.
Since that time, little work has been done to calculate population-level estimates of the proportion of IDUs initiating HAART and their virological responses to therapy. Because a growing number of IDUs will soon be at risk of HIV-related illness and death in many settings,4,5,6 it is critical to evaluate virological outcomes among IDUs who have presented for antiretroviral therapy. Therefore, we undertook the present study to characterize all HIV/AIDS patients who have initiated HAART in the province of British Columbia since 1996 and to evaluate virological response to treatment among patients with and without a history of injection drug use.
Methods
The dispensing of antiretroviral medications in British Columbia has been described in detail elsewhere.10,11 In brief, the HIV/AIDS Drug Treatment Program of the BC Centre for Excellence in HIV/AIDS remains the only source of free antiretroviral medications in the province, and less than 1% of HIV-infected British Columbians receive antiretrovirals from other sources.10 In June 1996 the Centre adopted antiretroviral therapy guidelines based on plasma viral loads, consistent with those put forward by the International AIDS Society-USA.12 The Centre's guidelines were revised in July 1997 to recommend triple-drug therapy for anyone with plasma HIV-1 RNA levels greater than 5000 copies/mL or CD4 cell counts below 500 cells/mL who had not previously received antiretroviral therapy.13 The Centre's HIV/AIDS Drug Treatment Program has received ethical approval from the University of British Columbia's Ethics Committee for Human Experimentation at its St. Paul's Hospital site, and the program conforms with the province's Freedom of Information and Protection of Privacy Act.
All HIV-infected men and women in the current study were entered into the HIV/AIDS Drug Treatment Program when they received their first prescription for antiretroviral agents. Any physician enrolling an HIV-infected person must complete a drug request enrolment form, which acts as a legal prescription and supplies baseline information, including past HIV-specific drug history, CD4 cell count, plasma HIV-1 RNA level, current drug prescriptions and data about the enrolling physician. At the time of the first refill, participants are asked to complete a survey, which elicits information on sociodemographic characteristics. The treating physicians are also asked to complete a clinical staging form that uses the World Health Organization clinical staging system.14 Thereafter, participants complete annual surveys on a volunteer basis. In the present study, we evaluated all HIV-1 infected men and women who had not taken antiretrovirals previously and who were first prescribed triple-drug antiretroviral therapy between Aug. 1, 1996, and July 31, 2000. The patients were followed until Mar. 31, 2002.
As an initial analysis, we evaluated the baseline demographic and clinical characteristics of patients with and without a history of injection drug use. This variable was defined on the basis of self-reports (through the annual participant survey) and through physician reports. To be conservative in our analysis, we considered any positive report of this risk behaviour at any time during follow-up indicative of a history of injection drug use. The categorical explanatory variables described below were analyzed with Pearson's χ2 test, and continuous variables were analyzed with the Wilcoxon rank-sum test.
We then evaluated time to suppression of plasma HIV-1 RNA after initiation of the first HAART regimen. As previously,9,15 suppression was defined as the first of at least 2 consecutive measurements of plasma HIV-1 RNA level of less than 500 copies/mL. Those who never achieved suppression were censored at the date of the last measurement of HIV-1 RNA before Mar. 31, 2002. Patients who achieved an HIV-1 RNA level of less than 500 copies/mL only once were not considered to have achieved suppression and hence were not included in this analysis.
We also evaluated the time to rebound of plasma HIV-1 RNA after initial virological suppression below 500 copies/mL. This analysis included any patient who achieved at least one RNA measure below 500 copies/mL. As previously,15 rebound of HIV-1 RNA was defined as the first of 2 consecutive measurements of plasma HIV-1 RNA level of 500 copies/mL or more after any measurement of less than 500 copies/mL. To be conservative, the rebound event was assumed to occur at the midpoint date between the last HIV-1 RNA measurement of less than 500 copies/mL and the first of the 2 consecutive measurements at least 500 copies/mL. For consistency with previous studies we evaluated only patients who experienced RNA suppression before 32 weeks after the initiation of therapy.15 Patients who experienced suppression but not rebound were censored as of Mar. 31, 2002, or, if follow-up ended before this date, as of the last HIV-1 RNA measurement.
For the 2 treatment outcomes (suppression and rebound), cumulative event rates were estimated by Kaplan–Meier methods. Cox regression was then used to calculate univariate and adjusted relative hazards (RHs) and 95% confidence intervals (CIs).16 The assumption of proportional hazards was validated by inspection of log (–log [survival function]) estimates against log time plots. We derived population-based estimates for the overall cohort and then, in subanalyses, examined adherent patients only.
The following salient baseline prognostic variables were examined: date of initiation of therapy, adherence, sex, age, use of protease inhibitor in the initial HAART regimen, prior clinical diagnosis of AIDS, physician experience, CD4 cell count, and log10-transformed plasma HIV-1 RNA level. We adjusted for the date of therapy initiation (on or after v. before July 31, 1997) in all multivariate analyses because this was the date when British Columbia's therapeutic guidelines for antiretroviral therapy were changed to recommend universal use of triple-drug regimens. The definition of adherence was based on the ratio of the period that the total amount of medication dispensed to the patient would last to the follow-up period during the first year on therapy, expressed as a percentage.17 We have previously demonstrated how this estimate strongly predicts virological response and mortality rate, and how it can be used to adjust for the potentially confounding effect of treatment interruption.9,18,19 Patients were defined a priori as nonadherent if they received antiretroviral medications for less than 95% of the follow-up period during the first year of therapy, as in previously published work.18 The definition of physician experience was also selected a priori on the basis of previous findings.20,21
For the multivariate analyses, we examined variables hypothesized a priori to be associated, either clinically or behaviourally, with virological response. These variables were first examined in univariate analyses to determine unadjusted RHs. Any variables associated with an event (suppression or rebound) in these univariate analyses (p < 0.05) were then entered into a fixed model. In addition, initial HAART regimen, baseline HIV-1 RNA level and CD4 cell count were entered into the final model because of their known relation to clinical and virological response.11,22 All tests of significance were 2-sided, with a p value of less than 0.05 indicating that an association was statistically significant.
Results
Between Aug. 1, 1996, and July 1, 2000, a total of 1583 participants who had never received antiretroviral therapy and who were 18 years of age or older began triple-drug therapy, consisting of 2 nucleoside reverse transcriptase inhibitors plus either a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor. Of these, 161 (10.2%) were excluded from this analysis because one or both of the baseline CD4 cell count and plasma HIV-1 RNA level had not been determined within 6 months before the start of the antiretroviral therapy. Those excluded from this analysis were more likely to be younger (p = 0.04) and taking protease inhibitors (p = 0.02). In the study population, 359 (25.2%) patients were identified as having a history of injection drug use, and 1063 (74.8%) were identified as non-IDUs.
Table 1 shows the results of univariate statistical comparisons of baseline clinical and sociodemographic characteristics. IDUs were less likely to be at least 95% adherent (p = 0.001), to be male (p = 0.001), to have a baseline clinical diagnosis of AIDS (p = 0.026) and to have a physician with HIV-related experience (p = 0.015). Conversely, IDUs were more likely to have received a protease inhibitor in the initial regimen (p = 0.026) and to have a higher CD4 cell count (p = 0.002). We detected no statistical difference between IDUs and non-IDUs with regard to date of therapy initiation, age and baseline HIV-1 RNA level.
Table 1
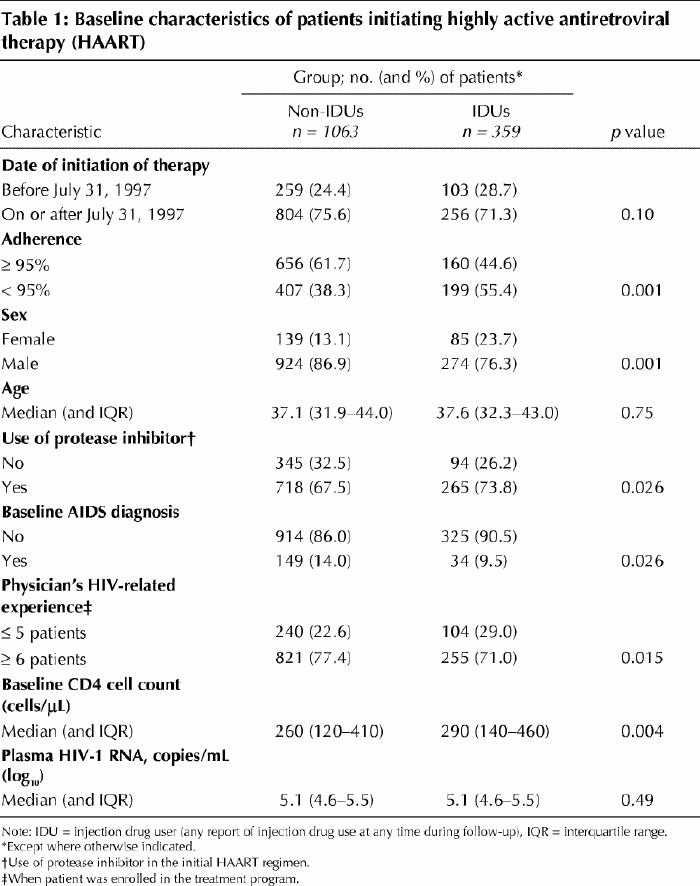
We observed marked differences between IDUs and non-IDUs in time to HIV-1 RNA suppression, as indicated by Kaplan–Meier estimates of the cumulative suppression rate for all patients (Fig. 1, left). The cumulative suppression rate at 12 months after initiation of HAART was 70.8% for non-IDUs and 51.4% for IDUs (log-rank, p < 0.001) (these values include people who achieved suppression before 12 months but who might not have been followed for the full 12-month period). However, a subanalysis showed that among the 816 patients (656 non-IDUs and 160 IDUs) who were defined as adherent, there was no difference between IDUs and non-IDUs in terms of HIV-1 RNA suppression (log-rank, p = 0.12) (Fig. 1, right).
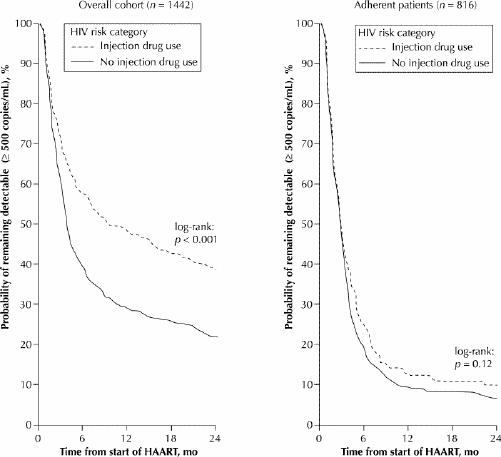
Fig. 1: Kaplan–Meier estimates of cumulative rates of suppression of plasma HIV RNA within the entire cohort of 1422 patients who initiated highly active antiretroviral therapy (HAART) during the study period (at left) and within the subpopulation of 816 patients who were defined as adherent (at right), according to history of injection drug use.
History of injection drug use was also strongly associated with lower rates of HIV-1 RNA suppression in univariate Cox regression analyses (RH 0.6, 95% CI 0.5–0.7). However, similar to our earlier analyses,9 rates of HIV-1 suppression were similar between non-IDUs and IDUs (adjusted RH 0.9, 95% CI 0.7–1.0) in a multivariate Cox model that adjusted for adherence, sex, age, protease inhibitor use, baseline CD4 cell count, HIV-1 RNA level and date of therapy initiation. In this model, adherence was strongly associated with suppression (adjusted RH 4.4, 95% CI 3.8–5.1). Similarly, injection drug use was not significant (adjusted RH 0.9, 95% CI 0.7–1.1) in subanalyses restricted to the 816 patients who were defined as adherent.
We also observed marked differences between IDUs and non-IDUs in time to HIV-1 RNA rebound, as indicated by Kaplan–Meier estimates of the cumulative rebound rate for the 970 patients who achieved HIV-1 RNA suppression, defined as less than 500 copies/mL at least once during follow-up (Fig. 2, left). Among patients who achieved suppression of HIV-1 RNA, the cumulative rebound rate at 12 months after initial suppression was 23.8% for non-IDUs and 34.7% for IDUs (log-rank, p < 0.001). However, a subanalysis showed that among the 716 adherent patients (577 non-IDUs and 139 IDUs) there was no difference between IDUs and non-IDUs in terms of HIV-1 RNA rebound (log rank, p = 0.12) (Fig. 2, right).
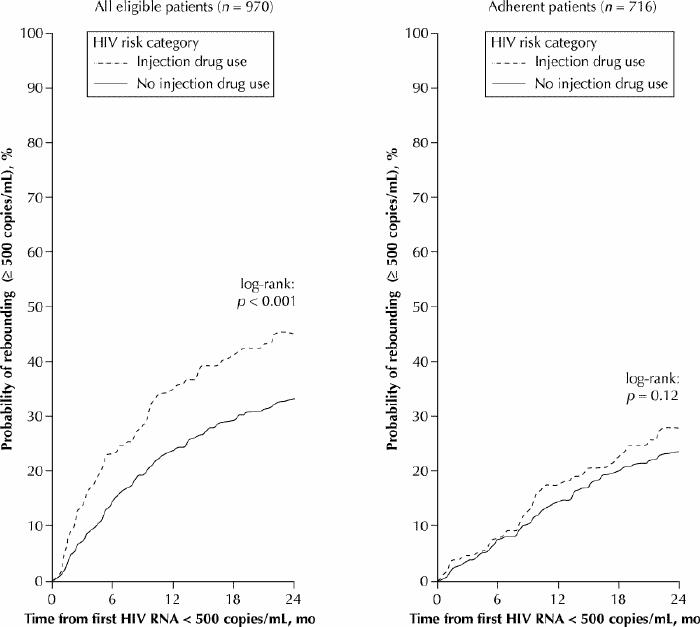
Fig. 2: Kaplan–Meier estimates of cumulative rates of rebound of plasma HIV RNA within the cohort of 970 patients who achieved suppression of HIV RNA (defined as viral count of less than 500 copies/mL at least once) during follow-up (at left) and within the subpopulation of 716 patients who were defined as adherent (at right), according to history of injection drug use.
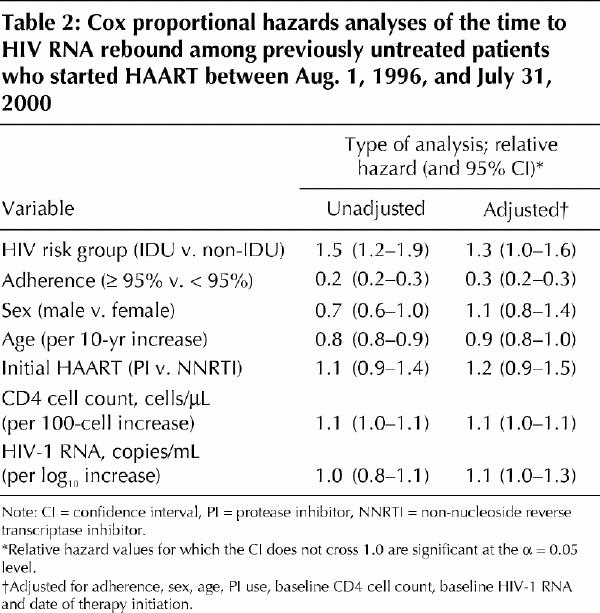
In univariate Cox regression analyses, IDUs had more rapid HIV-1 RNA rebound rates in univariate analyses (RH 1.5, 95% CI 1.2–1.9) (Table 2). However, as with suppression, the differences between IDUs and non-IDU were less pronounced in multivariate analyses (adjusted RH 1.3, 95% CI 1.0–1.6), with adjustment for adherence, sex, age, protease inhibitor use, baseline CD4 cell count, baseline HIV-1 RNA level and date of therapy initiation (Table 2). In this analysis, adherence was highly protective against HIV-1 RNA rebound (adjusted RH 0.3, 95% CI 0.2–0.3). As with HIV RNA suppression, history of injection drug use was also nonsignificant (adjusted RH 1.2, 95% CI 0.8–1.6) when this model was restricted to the 716 patients who were defined as adherent.
Table 2
Interpretation
We found that IDUs had markedly lower rates of plasma HIV-1 RNA suppression, and, among patients who achieved any HIV-1 RNA suppression, IDUs had markedly higher rates of rebound. However, these differences were explained by lower levels of adherence to HAART among the IDUs; the virological responses for the 2 groups were similar in adjusted analyses and restricted analyses that considered only adherent patients.
Several studies have demonstrated that the amount of HIV RNA circulating in the plasma is directly related to HIV disease progression,23,24,25 and our data indicate that IDUs in our setting will likely experience more rapid disease progression because of lower rates of virological response. However, we also found that the lower rates of virological response to HAART were primarily driven by lower levels of adherence among IDUs. Strategies that have helped to improve access and adherence to antiretrovirals among HIV-infected IDUs include directly observed therapy programs, access to medical services without appointment, on-site pharmacists at medical clinics and improved access to addiction treatment.26,27,28,29,30,31,32 Conversely, lower levels of HIV-related experience among physicians have been associated with worse access to therapy.31
With regard to tracking disease progression, the main limitation of the present study is that we examined only patients who actually initiated triple-drug therapy during the study period. To put the scale of this concern into perspective, consider that approximately 30% of the province's 5000 to 15 000 IDUs have HIV infection,33,34 of whom only 359 initiated triple-drug therapy during this period. Other limitations, which probably resulted in strong conservative biases in our analyses, are the fact that injection drug use is a stigmatized behaviour that may be underreported by IDUs and the fact that some patients with a history of injection drug use might have become abstinent during follow-up.35 Similarly, people who use non-injection illicit drugs, such as crack cocaine, may be at similar risk of poor adherence to HAART,36 but these were included in the comparison group in the present study. In addition, although using refill compliance as a measure of adherence has been previously validated,9,17,18,19 there might have been differences in adherence levels between IDUs and non-IDUs, even among patients who were defined as adherent;35 this again represents a conservative bias. Finally, because we do not have population-based data on coinfection with hepatitis C, this variable could not be evaluated in the present study.21
In summary, we found that IDUs had markedly lower rates of virological suppression and higher rates of virological rebound. Because much of the difference in virological response rates is explained by lower levels of adherence to HAART, programs that have been shown to improve adherence with antiretroviral therapy, such as addiction treatment strategies,32,37 should be expanded. Our findings have implications for public health and medical service use because, given what is known about the HIV epidemic among IDUs in British Columbia,7,33,34 we can expect that IDUs will soon be experiencing high levels of illness and death as a result of lower levels of adherence with therapy.
Footnotes
This article has been peer reviewed.
Contributors: Evan Wood and Robert Hogg designed the study. Evan Wood conducted the statistical analyses and prepared the first draft of the manuscript. All coauthors contributed substantively to the final design of the study and its revisions, analysis of the data and final interpretation of the results and also contributed to the various drafts of the manuscript.
Acknowledgments: Evan Wood was supported by the Canadian Institutes for Health Research, the BC Heath Research Foundation and the Michael Smith Foundation for Health Research. This work was also supported by the Michael Smith Foundation for Health Research through a Career Investigator Award and by the Canadian Institutes of Health Research through an Investigator Award to Robert Hogg. We thank Bonnie Devlin, Chandra Lips, Diane Campbell, Elizabeth Ferris, Nada Gataric, Kelly Hsu, Myrna Reginaldo and Peter Vann for their administrative assistance.
Competing interests: None declared.
Correspondence to: Dr. Evan Wood, Division of Epidemiology and Population Health, British Columbia Centre for Excellence in HIV/AIDS, 608-1081 Burrard St., Vancouver BC V6Z 1Y6; fax 604 806-9044; ewood@hivnet.ubc.ca
References
- 1.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 1997;337(11):725-33. [DOI] [PubMed]
- 2.Montaner JS, Reiss P, Cooper D, Verra S, Harris M, Conway B, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. Italy, The Netherlands, Canada and Australia Study. JAMA 1998;279(12):930-7. [DOI] [PubMed]
- 3.Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard GB, Hammer SM, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society—USA Panel. JAMA 2000;283(3):381-90. [DOI] [PubMed]
- 4.Karon JM, Fleming PL, Steketee RW, De Cock KM. HIV in the United States at the turn of the century: an epidemic in transition. Am J Public Health 2001; 91(7):1060-8. [DOI] [PMC free article] [PubMed]
- 5.Anderson KH, Mitchell JM. Differential access in the receipt of antiretroviral drugs for the treatment of AIDS and its implications for survival. Arch Intern Med 2000;160(20):3114-20. [DOI] [PubMed]
- 6.Rosenberg PS. Scope of the AIDS epidemic in the United States. Science 1995;270(5240):1372-5. [DOI] [PubMed]
- 7.Strathdee SA, Patrick DM, Currie SL, Cornelisse PG, Rekart ML, Montaner JS, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS 1997;11(8):F59-65. [DOI] [PubMed]
- 8.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Bupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med 1997;126(12):946-54. [DOI] [PubMed]
- 9.Palepu A, Yip B, Miller C, Strathdee SA, O'Shaughnessy MV, Montaner JS, et al. Factors associated with the response to antiretroviral therapy among HIV-infected patients with and without a history of injection drug use. AIDS 2001;15:423-4. [DOI] [PubMed]
- 10.Wood E, Schechter MT, Tyndall MW, Montaner JS, O'Shaughnessy MV, Hogg RS. Antiretroviral medication use among injection drug users: two potential futures. AIDS 2000;14(9):1229-35. [DOI] [PubMed]
- 11.Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O'Shaughnessy MV, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA 2001;286(20):2568-77. [DOI] [PubMed]
- 12.Carpenter CC, Fischl MA, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, et al. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society—USA. JAMA 1996;276(2):146-54. [PubMed]
- 13.Carpenter CC, Fischl MA, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, et al. Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society—USA Panel. JAMA 1997;277(24):1962-9. [PubMed]
- 14.World Health Organization. Acquired immune deficiency syndrome (AIDS): interim proposal for a WHO staging system for HIV infection and disease. Wkly Epidemiol Rec 1990;65:221-8. [PubMed]
- 15.Phillips AN, Staszewski S, Weber R, Kirk O, Francioli P, Miller V, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. JAMA 2001;286(20):2560-7. [DOI] [PubMed]
- 16.Cox DR. Regression models and life tables. J R Stat SocSer B 1972;34:187-202.
- 17.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol 1997;50(1):105-16. [DOI] [PubMed]
- 18.Low-Beer S, Yip B, O'Shaughnessy MV, Hogg RS, Montaner JS. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr 2000;23(4):360-1. [DOI] [PubMed]
- 19.Hogg RS, Heath KV, Bangsberg D, Yip B, Press N, O'Shaughnessy MV, et al. Intermittent use of triple combination therapy is predictive of mortality at baseline and after one year of follow-up. AIDS 2002;16(7):1051-8. [DOI] [PubMed]
- 20.Kitahata MM, Koepsell TD, Deyo RA, Maxwell CL, Dodge WT, Wagner EH. Physicians' experience with the acquired immunodeficiency syndrome as a factor in patients' survival. N Engl J Med 1996;334(11):701-6. [DOI] [PubMed]
- 21.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. Is there a baseline CD4 cell count that precludes a survival response to modern antiretroviral therapy? AIDS 2003;17(5):711-20. [DOI] [PubMed]
- 22.Ghani AC, Henley WE, Donnelly CA, Mayer S, Anderson RM. Comparison of the effectiveness of non-nucleoside reverse transcriptase inhibitor-containing and protease inhibitor-containing regimens using observational databases. AIDS 2001;15(9):1133-42. [DOI] [PubMed]
- 23.Mellors JW, Rinaldo CR Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996;272(5265):1167-70. [DOI] [PubMed]
- 24.Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet 1999;353(9156):863-8. [DOI] [PubMed]
- 25.Lundgren JD, Mocroft A, Gatell JM, Ledergerber B, D'Aminio Monforte A, Hermans P, et al. A clinically prognostic scoring system for patients receiving highly active antiretroviral therapy: results from the EuroSIDA study. J Infect Dis 2002;185(2):178-87. [DOI] [PubMed]
- 26.Mitty JA, Stone VE, Sands M, Macalino G, Flanigan T. Directly observed therapy for the treatment of people with human immunodeficiency virus infection: a work in progress. Clin Infect Dis 2002;34(7):984-90. [DOI] [PubMed]
- 27.Sherer R, Stieglitz K, Narra J, Jasek J, Green L, Moore B, et al. HIV multidisciplinary teams work: support services improve access to and retention in HIV primary care. AIDS Care 2002;14(Suppl 1):S31-44. [DOI] [PubMed]
- 28.Celentano DD, Vlahov D, Cohn S, Shadle VM, Obasanjo O, Moore RD. Self-reported antiretroviral therapy in injection drug users. JAMA 1998;280(6):544-6. [DOI] [PubMed]
- 29.Andersen R, Bozzette S, Shapiro M, St Clair P, Morton S, Crystal S, et al. Access of vulnerable groups to antiretroviral therapy among persons in care for HIV disease in the United States. HCSUS Consortium. HIV Cost and Services Utilization Study. Health Serv Res 2000;35(2):389-416. [PMC free article] [PubMed]
- 30.Bamberger JD, Unick J, Klein P, Fraser M, Chesney M, Katz MH. Helping the urban poor stay with antiretroviral HIV drug therapy. Am J Public Health 2000; 90(5):699-701. [DOI] [PMC free article] [PubMed]
- 31.Strathdee SA, Palepu A, Cornelisse PG, Yip B, O'Shaughnessy MV, Montaner JS, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA 1998;280(6):547-9. [DOI] [PubMed]
- 32.Antela A, Casado JL, Gonzalez MJ, Perez P, Perez-Elias MJ, Montilla P, et al. Influence of a methadone maintenance programme on the improved outcome of a cohort of injecting drug users with advanced HIV disease. AIDS 1997;11(11):1405-6. [PubMed]
- 33.Spittal PM, Craib KJ, Wood E, Laliberté N, Li K, Tyndall MW, et al. Risk factors for elevated HIV incidence rates among female injection drug users in Vancouver. CMAJ 2002;166(7):894-9. [PMC free article] [PubMed]
- 34.Craib KJ, Spittal PM, Wood E, Laliberté N, Hogg RS, Li K, et al. Risk factors for elevated HIV incidence among Aboriginal injection drug users in Vancouver. CMAJ 2003;168(1):19-24. [PMC free article] [PubMed]
- 35.Palepu A, Tyndall MW, Yip B, O'Shaughnessy MV, Hogg RS, Montaner JS. Impaired virologic response to highly active antiretroviral therapy associated with ongoing injection drug use. J Acquir Immune Defic Syndr 2003;32(5):522-6. [DOI] [PubMed]
- 36.Edlin BR, Irwin KL, Faruque S, McCoy CD, Word C, Serrano Y, et al. Intersecting epidemics — crack cocaine use and HIV infection among inner-city young adults. Multicenter Crack Cocaine and HIV Infection Study Team. N Engl J Med 1994;331(21):1422-7. [DOI] [PubMed]
- 37.Rehm J, Gschwend P, Steffen T, Gutzwiller F, Dobler-Mikola A, Uchtenhagen A. Feasibility, safety, and efficacy of injectable heroin prescription for refractory opioid addicts: a follow-up study. Lancet 2001;358(9291):1417-23 [DOI] [PubMed]


