Abstract
Pavlovian conditioning in Hermissenda consists of pairing light, the conditioned stimulus (CS) with activation of statocyst hair cells, the unconditioned stimulus (US). Conditioning produces CS-elicited foot shortening and inhibition of light-elicited locomotion, the two conditioned responses (CRs). Conditioning correlates have been identified in the primary sensory neurons (photoreceptors) of the CS pathway, interneurons that receive monosynaptic input from identified photoreceptors, and putative pedal motor neurons. While cellular mechanisms of acquisition produced by the synaptic interaction between the CS and US pathways are well-documented, little is known about the mechanisms responsible for the generation or expression of the CR. Here we show that in conditioned animals light reduced tonic firing of ciliary activating pedal neurons (VP1) below their pre-CS baseline levels. In contrast, pseudorandom controls expressed a significant increase in CS-elicited tonic firing of VP1 as compared to pre-CS baseline activity. Identified interneurons in the visual pathway that have established polysynaptic connections with VP1 were examined in conditioned animals and pseudorandom controls. Depolarization of identified type Ie interneurons with extrinsic current elicited a significant increase in IPSPs recorded in VP1 pedal neurons of conditioned animals as compared with pseudorandom controls. Conditioning also enhanced intrinsic excitability of type Ie interneurons of conditioned animals as compared to pseudorandom controls. Light evoked a modest increase in IPSP frequency in VP1 of conditioned preparations and a significant decrease in IPSP frequency in VP1 of pseudorandom controls. Our results show that a combination of synaptic facilitation and intrinsic enhanced excitability in identified components of the CS pathway may explain light-elicited inhibition of locomotion in conditioned animals.
Pavlovian conditioning in Hermissenda results in conditioned stimulus (CS)-elicited inhibition of phototaxis (Crow and Alkon 1978; Crow and Offenbach 1983) and CS-elicited foot contraction (Lederhendler et al. 1986). While these two motor acts appear dissimilar, inhibition of CS-elicited ciliary locomotion and CS-elicited foot contraction may synergistically interact to contribute to the inhibition of phototactic behavior observed in conditioned animals. While some progress has been made in the identification of foot contraction motor neurons (Goh and Alkon 1984) and cellular neurophysiological correlates of conditioning in a contractile pedal motor neuron (Goh et al. 1985), little is known regarding cellular plasticity in the neural network supporting ciliary locomotion in conditioned Hermissenda. Recently, pedal neurons activating cilia on the anterior foot have been identified and synaptic connections between type I, II, and III interneurons and VP1 ciliary activating pedal neurons have been established (Crow and Tian 2003).
In this report we show that Pavlovian conditioning results in light-elicited inhibition of the spike activity of VP1 pedal neurons as compared to pseudorandom controls. Light elicited an increase in tonic spike activity of pseudorandom controls that was similar to light-evoked increases in tonic spike activity detected in normal untrained animals. In addition, light elicited an increase in IPSP frequency in VP1 pedal neurons of conditioned preparations, and IPSP frequency in VP1 pedal neurons evoked by depolarization of identified type Ie interneurons was significantly increased in conditioned animals as compared to pseudorandom controls. Conditioning also produced an enhancement of intrinsic excitability of type Ie interneurons. The previously reported facilitation of the amplitude of monosynaptic and complex EPSPs in type Ie interneurons of conditioned animals in conjunction with the intrinsic enhanced excitability of type Ie interneurons detected following conditioning contributes to the inhibition of ciliary locomotion elicited by light. The diversity of polysensory interneuronal synaptic projections to the motor network supporting ciliary locomotion and foot movement may explain how Pavlovian conditioning can modify components of a circuit that must also support normal visual influences of motor behavior.
RESULTS
A total of 100 animals were used in the behavioral experiments. The conditioned group consisted of 50 animals, and 50 animals served in the pseudorandom control groups. Animals were tested 24 h postconditioning followed by isolation of the circumesophageal nervous system and collection of electrophysiological data from type I interneurons and VP1 pedal neurons. Since not all isolated nervous systems resulted in the identification and successful penetration of interneurons and pedal neurons, the sample sizes for the neural correlates were smaller than the total number of animals included in the conditioned and pseudorandom control groups. However, the suppression ratios for these groups were not significantly different from the larger groups.
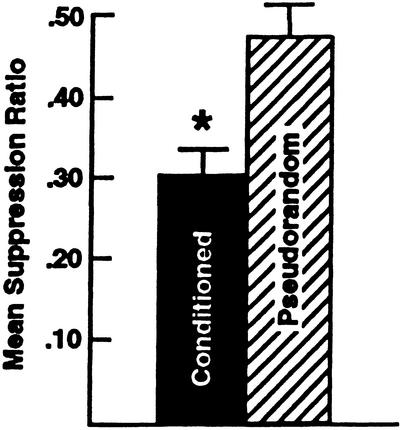
The mean suppression ratios for the conditioned and pseudorandom control groups are shown in Figure 1. As consistent with previous reports, the results of the statistical analysis showed that conditioning produced significant behavioral suppression (X̄ = 0.30 ±0.03) as compared with the pseudorandom control group (X̄ = 0.47 ±0.04) (t98 = 3.9; P < .001).
Figure 1.
Mean suppression ratios ±SEM for conditioned (n = 50) and pseudorandom controls (n = 50). Conditioning produced statistically significant suppression of light-elicited locomotion compared with pseudorandom controls (*P < 0.001).
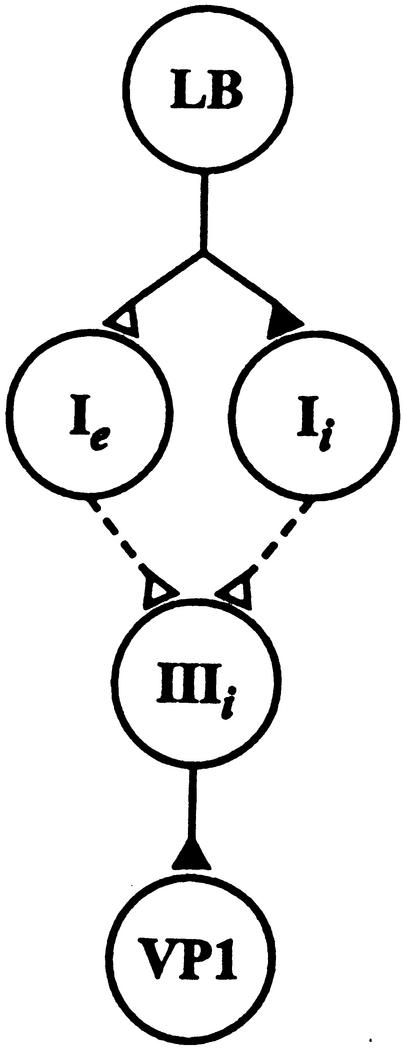
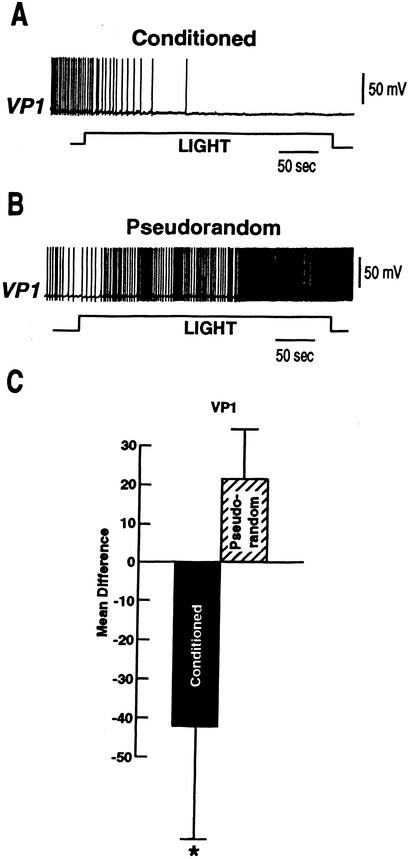
A diagram depicting components of the CS pathway projecting to ciliary activating motor neurons is shown in Figure 2. The diagram represents identified connections between a photoreceptor and motor neurons supporting ciliary locomotion. For studies of the motor system in conditioned animals, we first examined changes in the tonic spike activity of VP1 pedal neurons duringa5min period of illumination of the eyes. Activity was expressed as difference scores wherea5min period of baseline spike activity in the dark that immediately preceded the 5 min light step was subtracted from tonic spike activity evoked in light. Examples of intracellular recordings of spike activity in VP1 during the light from a conditioned preparation and pseudorandom control are shown in Figure 3A,B. Summary group data depicting mean difference scores for the conditioned group and pseudorandom controls is shown in Figure 3C. The results showed that light elicited an increase in the tonic spike activity recorded in VP1 for the pseudorandom control group. In contrast, the spike activity of VP1 showed a decrease below baseline during the light for the conditioned group (Fig. 3A). A comparison of the difference scores shown in Fig. 3C for the conditioned group (n = 10) and pseudorandom controls (n = 9) showed that they were statistically significant (t17 = 2.1; P < .05). These results show that light inhibits the tonic spike activity of VP1 pedal neurons in conditioned animals.
Figure 2.
Components of the CS pathway involved in visually mediated ciliary locomotion in Hermissenda. Only connections with a single photoreceptor are shown. The diagram shows a monosynaptic connection between a lateral B-photoreceptor and type Ie and Ii interneurons. The dashed lines between the type Ie and Ii and type IIIi interneurons indicate a polysynaptic pathway with unidentified interneurons. The monosynaptic connections between type IIIi interneurons and VP1 have been previously established (Crow and Tian 2003). Filled triangles denoted inhibitory synapses, open excitatory.
Figure 3.
Examples of light-elicited changes in spike activity for conditioned animals and pseudorandom controls. (A) Light-elicited decrease in the spike activity recorded in pedal neuron VP1 from a conditioned animal. (B) Light-elicited increase in the spike activity recorded in VP1 from a pseudorandom control. (C) Group data depicting the mean difference in VP1 spike activity evoked in 5 min of light and a 5-min period in the dark immediately preceding light onset collected from conditioned animals ((n = 10) and pseudorandom controls (n = 9; *P < .05).
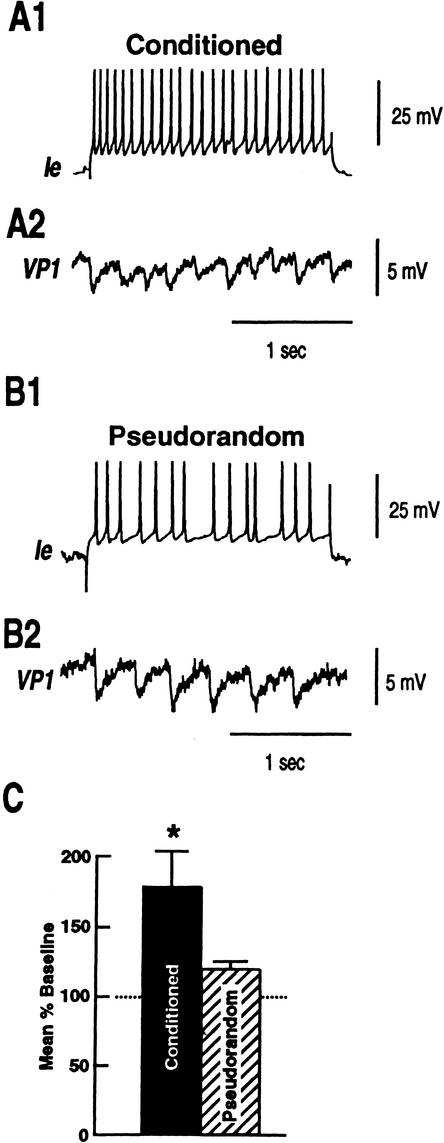
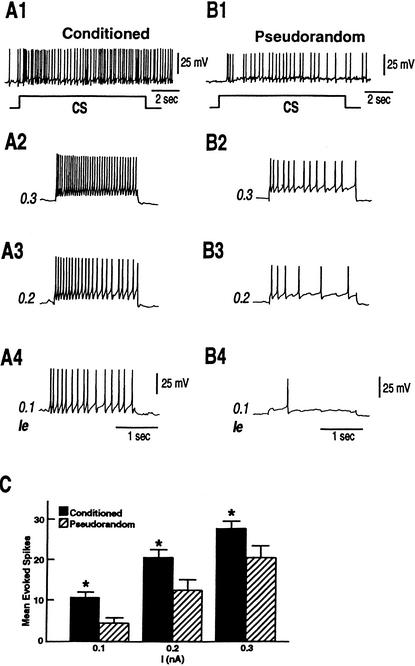
Previous work has shown that VP1 receives polysynaptic input from components of the visual system, identified type Ie interneurons. We examined the source of the inhibition of VP1 by recording simultaneously from type Ie interneurons and pedal neuron VP1. As shown in the examples of Figure 4, depolarization of a type Ie interneuron in a conditioned animal with a 2 sec 0.3 nA extrinsic current pulse evoked more IPSPs recorded in VP1 (Fig. 4A2) as compared to the example from a pseudorandom control (Fig. 4B2). The group data summarized in Figure 4C showed that mean evoked IPSP frequency relative to baseline IPSP activity was greater in VP1 pedal neurons of conditioned animals (n = 12) (X̄ = 1.8 ±.26) as compared to the pseudorandom controls (n = 9) (X̄ = 1.2 ±.05). The difference in current evoked IPSP frequency between the conditioned and pseudorandom controls was statistically significant (t19 = 1.97; p < .05). Since the depolarizing current pulse elicited more spikes in type Ie interneurons from conditioned preparations, the increase in the number of IPSPs evoked by stimulation of type Ie interneurons from conditioned preparations could be due to intrinsic changes in excitability of type Ie interneurons. We examined the excitability of type Ie interneurons further with three levels (0.1, 0.2, 0.3 nA) of extrinsic depolarization current pulses. The examples in Figure 5A1,B1 show CS-elicited increases in spike activity in identified type Ie interneurons from a conditioned preparation and a pseudorandom control. As shown previously (Crow and Tian 2002b) the CS elicits more spikes in type Ie interneurons of conditioned animals. In addition, as shown in the examples in Figure 5A2–A4, type Ie interneurons of conditioned preparations (n = 10) expressed more action potentials evoked by the three extrinsic current pulses of increasing magnitude as compared to pseudorandom controls (Fig. 5B2–B4; n = 12). The analysis of the group data shown in Figure 5C revealed that the three levels of extrinsic current pulses evoked significantly more spikes in conditioned preparations as compared with the pseudorandom controls. The outcome of the ANOVA revealed an overall significant difference between the conditioned group and the pseudorandom controls (F1,20 = 7.8; P < .01). The overall effect of current levels for both groups was also statistically significant (F2,140 = 151.3; P < .001). The interaction between the different treatments and current level was not statistically significant (F2,20 = .41; NS). The results showed that excitability was enhanced at all current levels for the conditioned group relative to the pseudorandom controls (q = 5.9, q = 5.5, q = 5.2; P < .05). These results indicate that one contribution to the current evoked increase in IPSP frequency detected in VP1 pedal neurons, produced by depolarization of type Ie interneurons, is the intrinsic enhanced excitability of type Ie interneurons of conditioned animals.
Figure 4.
Examples of depolarizing current elicited IPSPs in VP1 for conditioned animals and pseudorandom controls. (A1)An example from a conditioned animal of spikes evoked bya2sec0.3 nA depolarizing current pulse applied to an identified type Ie interneuron. (A2) An increase in the number of IPSPs relative to baseline recorded in VP1 elicited by depolarization of Ie.(B1)An example from a pseudorandom control animal of spikes evoked by a 2 sec 0.3 nA depolarizing current pulse applied to an identified type Ie interneuron. (B2) IPSPs in VP1 evoked by depolarization of Ie from a pseudorandom control. (C) Group data depicting the mean percent increase from baseline in VP1 IPSPs elicited by depolarization of identified type Ie interneurons from conditioned animals (n = 12) and pseudorandom controls (n = 9; *P < .05).
Figure 5.
Conditioning produces enhanced excitability of identified type Ie interneurons as compared to pseudorandom controls. (A1) An example of the CS-elicited depolarization and increase in spike frequency recorded in a type Ie interneuron from a conditioned animal and a pseudorandom control (B1). (A2–A4) Excitability was assessed in the type Ie interneuron shown in (A1) from a conditioned animal with 2-sec depolarizing extrinsic current pulses of increasing intensity (0.1 nA–0.3 nA). (B2–B4) Excitability of the type Ie interneuron shown in (B1) from a pseudorandom control assessed with 2-sec depolarizing extrinsic current pulse of increasing intensity (0.1 nA–0.3 nA). (C) Group data showing the mean evoked spikes recorded in type Ie interneurons from conditioned animals (n = 10) and pseudorandom controls (n = 12) for the three different depolarizing current levels (*P<.05).
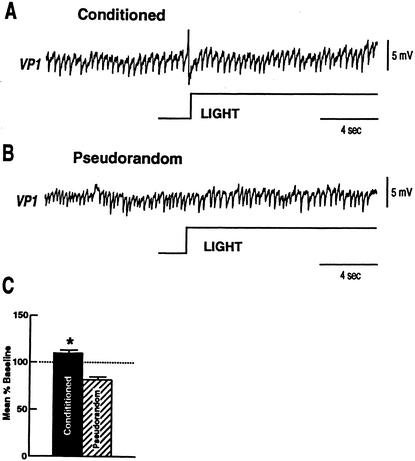
We next examined the effect of light on the synaptic activity of VP1 pedal neurons by measuring IPSP frequency duringa1min period of illumination.A1min period of spontaneous IPSPs recorded from VP1 in the dark was used to establish a baseline and changes in IPSP frequency during light were expressed as a percent of baseline IPSP activity. Examples of VP1 IPSPs occurring during light for a conditioned preparation and a pseudorandom control are shown in Figure 6A,B. Light elicited a modest increase in IPSP frequency for the conditioned preparation and a typical decrease in IPSP frequency for the pseudorandom control. The group data comparing the mean percent of baseline for IPSPs elicited during the light step for conditioned (X̄ = 1.09) (n = 12) and pseudorandom controls (X̄ = .84) (n = 11) is shown in Figure 6C. The statistical analysis showed that the conditioned group was significantly different from the pseudorandom controls (t21 = 3.7; p < .001). These results show that light elicits a disinhibition of VP1 pedal neurons in pseudorandom controls as compared to the conditioned group. We have shown previously that a characteristic of the synaptic activity of VP1 is a relatively high occurrence of spontaneous IPSPs in the dark that are decreased by light (Crow and Tian 2003). Moreover, it has been shown previously that light-evoked disinhibition of VP1 is due in part to the light evoked complex IPSP detected in type Ii and IIi interneurons, and light-evoked excitation of type IIe interneurons (Crow and Tian 2003). Our results support the hypothesis that enhanced disinhibition of VP1 mediated by the increased amplitude of complex IPSPs in type Ii and IIi interneurons produced by conditioning is blocked by the increased inhibition of VP1 produced by intrinsic enhanced excitability of type Ie interneurons and synaptic facilitation of type Ie monosynaptic EPSPs. Recordings from normal control preparations revealed that light typically produced a 15%–20% decrease in IPSP frequency in VP1 pedal neurons (Crow and Tian 2003). Therefore, light-evoked disinhibition of VP1 neurons is one mechanism for activating ciliary locomotion in Hermissenda. The results presented in this report indicate that conditioning produces an enhancement of light-evoked inhibition of VP1 ciliary activating pedal neurons.
Figure 6.
Light-elicited changes in VP1 IPSP frequency in conditioned animals and pseudorandom controls. (A) Example of a light-elicited increase in VP1 IPSPs relative to baseline from a conditioned preparation. (B) Example of a light-elicited decrease in VP1 IPSPs relative to baseline from a pseudorandom control preparation. (C) Group data depicting the mean percent change from baseline during light-evoked (1 min) IPSP frequency for conditioned animals (n = 12) and pseudorandom controls (n = 11) (*P < .001).
DISCUSSION
We have examined changes in light-elicited spike activity and IPSP frequency of ciliary activating pedal neurons, and assessed changes in intrinsic excitability of type Ie interneurons in classically conditioned Hermissenda. In conditioned preparations, light resulted in a significant decrease below baseline in spike activity of pedal neurons VP1. In contrast, light elicited an increase in spike activity of VP1 pedal neurons in pseudorandom controls. Previous research has shown that pedal nerves P1 and P2 contain the axons of pedal neurons responsible for generating locomotion in Hermissenda (Richards and Farley 1987). The anterior region of the foot is innervated by pedal nerve P2 (Richards and Farley 1987; Crow and Tian 2003). Intracellular recordings from VP1 neurons in conjunction with suction electrode recordings from nerve P2 have verified that VP1 neurons project axonal processes in pedal nerve P2 (Crow and Tian 2003). Our results showing that light evokes a decrease in VP1 spike activity below baseline are consistent with the previously published observations showing that conditioning reduced light-elicited extracellular spike activity recorded from pedal nerves P1 and P2, and a random control procedure produced light-elicited increases in extracellular spike activity of pedal nerves P1 and P2 (Richards and Farley 1987).
The visual and statocyst pathways are well-characterized in Hermissenda (Alkon and Fuortes 1972; Alkon 1973a,1973b; Alkon et al. 1978; Crow et al. 1979). Previous studies have made some progress in the identification of light-responsive putative motor neurons in the pedal ganglia (Jerussi and Alkon 1981; Goh and Alkon 1984; Goh et al. 1985; Richards and Farley 1987; Hodgson and Crow 1991; 1992). Components of the neural circuit responsible for generating ciliary locomotion in Hermissenda have now been identified (Crow and Tian 2000; 2003; see Fig. 2). Pedal neuron VP1 has been shown to activate cilia on the sole of the anterior foot (Crow and Tian 2003). We have shown that light stimulation of the visual system has two effects upon the activity of pedal neuron VP1 that is mediated by several pathways. First, light inhibits type Ii and IIi interneurons, which results in disinhibition of VP1 pedal neurons. Second, light-elicited depolarization of type IIe interneurons also disinhibits VP1 neurons. VP1 pedal neurons receive monosynaptic inhibitory input from type IIIi interneurons. Type IIIi interneurons are inhibited by type IIe interneurons and excited by type Ii and IIi interneurons. Therefore, light-elicited increases in tonic spike activity of VP1 pedal neurons is modulated by inhibition of type Ii and IIi interneurons, which results in a decrease in VP1 IPSPs generated by type IIIi interneurons. Consistent with the proposed circuitry generating ciliary locomotion and its modulation by light, we found that conditioning produced an increase in light-elicited IPSPs in VP1 pedal neurons. In contrast, light-elicited disinhibition of VP1 was observed in pseudorandom controls. Interestingly, stimulation of identified type Ie interneurons with depolarizing extrinsic current produced a significant increase in IPSP frequency above baseline in VP1 of conditioned animals as compared with pseudorandom controls. The increase in VP1 IPSP frequency produced by depolarization of type Ie interneurons can be explained in part by the enhanced excitability of type Ie interneurons of conditioned animals. We previously reported that conditioning produced a CS-elicited increase in spike activity of type Ie interneurons (Crow and Tian 2002b). Therefore, the CS-elicited increase in type Ie interneuron spike activity is the result of both increased synaptic facilitation of the synaptic connections between photoreceptors and type Ie interneurons, CS-elicited increased spike activity of photoreceptors, and enhanced intrinsic excitability of type Ie interneurons described in this report. This indicates that an important component of conditioning-associated plasticity in the network responsible for generating the CR is in the type I interneurons. The present results may explain how CS-elicited enhancement of the generator potential, CS-elicited enhancement of complex PSP amplitude of interneurons, and facilitation of monosynaptic PSPs in interneurons of conditioned animals produces behavioral inhibition. Inhibition of light-elicited ciliary locomotion may be caused by conditioning-dependent increases in IPSP frequency in VP1 elicited by enhanced depolarization and increased spike activity of Ie interneurons, resulting in an increase in VP1 IPSP frequency and a decrease in the tonic spike activity of VP1 pedal neurons.
In a number of invertebrates, interneurons have been identified as sites of plasticity. A network of interneurons has been shown to contribute to olfactory learning and olfactory discrimination in Limax (Kleinfeld et al. 1994; Gelperin and Flores 1997; Ermentrout et al. 2001). Modifications in interneurons in the feeding circuit of Lymnaea have been identified in conditioned animals (Benjamin et al. 2000). In Aplysia, interneurons have been proposed as sites of plasticity in habituation and sensitization (Cleary et al. 1995). A correlate of food avoidance learning in Pleurobranchaea is a reduction in EPSP amplitude in CCD interneurons of the feeding circuit (Kovac et al. 1986). In addition, intrinsic changes in excitability of leech S cells occur with nonassociative learning of the shortening reflex (Burrell et al. 2001), and Retzius cells express a correlate of CS–US predictability (Sahley and Crow 1998).
Enhancement of excitability in cortex and hippocampus is a general characteristic of Pavlovian conditioning in mammals (Woody and Engel 1972; Woody et al. 1976; Disterhoft et al. 1986; Moyer et al. 1996; Thompson et al. 1996). A number of invertebrate preparations also exhibit enhanced excitability in identified neurons of conditioned animals (for review see Sahley and Crow 1998). Studies of Pavlovian conditioning of Hermissenda have identified several sites of plasticity in the CS pathway consisting of modifications in both excitability and synaptic efficacy (Crow and Alkon 1980; Farley and Alkon 1982; West et al. 1982; Frysztak and Crow 1993, 1994, 1997; Gandhi and Matzel 2000; Crow and Tian 2002b). Voltage-clamp experiments conducted on type B photoreceptors isolated by axotomy after conditioning showed that voltage (IA, ICa) and Ca2+-dependent (IK,Ca) currents are reduced (Alkon et al. 1982, 1985; Collin et al. 1988). The net effect of modifications in the different extrinsic membrane conductances of sensory neurons(photoreceptors) could explain the CS-elicited enhancement of the generator potential and CS-evoked increase in spike activity of the sensory neurons from conditioned animals. These changes in excitability in conjunction with synaptic facilitation of the monosynaptic B-photoreceptor-type I interneuron PSP may explain the enhancement of complex PSPs in type Ie and Ii interneurons (Crow and Tian 2002b). The observation described in this report of an enhancement of excitability of type Ie interneurons with conditioning is the first evidence for intrinsic changes in interneurons in Hermissenda produced by Pavlovian conditioning. Taken collectively, the plasticity in the various components of the CS pathway provides an explanation for light-elicited inhibition of ciliary locomotion in conditioned animals.
One of the characteristics of Pavlovian conditioning is the formation of a new response (CR) elicited by the CS after training. In Hermissenda the response evoked by the CS before conditioning is different from both the UR and CR. Since a new response, inhibition of locomotion and foot shortening (CRs), is elicited by the CS after training, conditioning in Hermissenda is not an example of reflex potentiation (e.g. Schreurs 1989). However, since the CS-elicits an increase in spike activity, synaptic facilitation, and an increased amplitude of complex PSPs in components of the CS pathway, it is not clear how such changes could be expressed in the inhibition of behavior. Interestingly, the present findings suggest that the balance of synaptic connections between type Ie,Ii interneurons and VP1 pedal neurons are functionally modified by Pavlovian conditioning. Therefore, CS-evoked activation of type Ie interneurons and inhibition of type Ii interneurons produces a net inhibition of the tonic spike activity of cilliary activating VP1 pedal neurons normally excited by light.
MATERIALS AND METHODS
Animals
Adult Hermissenda crassicornis were used in the experiments. The animals were obtained from Sea Life Supply, and maintained in closed artificial seawater aquaria at 14 ±1°C on a 12-h light, 12-h dark cycle. Behavioral training, testing and electrophysiological procedures were performed during the light phase of the light/dark cycle.
Baseline Test of Phototactic Behavior
The details of the conditioning procedure and methods for testing phototactic behavior have been described in detail in previous publications (Crow and Alkon 1978; Crow and Offenbach 1983) and will be described only briefly in this report. Animals were tested prior to training to determine baseline latencies to initiate locomotion in response to a test light. Animals that did not respond within a 20 min criterion period during the pretraining measurements were not used in the conditioning experiments. Previous research has shown that the increase in the time taken by the animals to locomote into a test light can be accounted for by an increase in the latency to initiate locomotion (Crow and Offenbach 1983). Animals were placed into glass tubes 228-mm long filled with artificial seawater. A foam plug inserted through an opening confined the animal to one end of the tube. The tubes were attached by spring clips to a modified turntable enclosed in an incubator maintained at 15°C. Animals were dark-adapted for 12 min prior to testing phototactic behavior. A light spot (10-4 watts/cm2, white light) was projected onto the center of the turntable, illuminating a circular area 15–16 cm in diameter. The elapsed times to initiate locomotion in the presence of the test light were recorded when a Hermissenda moved between an infrared emitter and a phototransistor located at the starting end of each glass tube. When the infrared beam was interrupted, a free running digital clock was turned off and the time recorded for later data analysis.
Conditioning Procedure
Following baseline testing, animals were randomly assigned to the conditioned group or pseudorandom control group. The conditioning phase consisted of 50 trials of the 10 sec CS (light) paired with the unconditioned stimulus (US; high speed rotation; mean ITI = 2.5 min), presented each day for 2 consecutive days. The intensity of the CS was the same as the test light used to establish baseline responding for phototactic behavior during the pretest condition. The pseudorandom control group received a total of 100 trials of the CS and US (50 trials each day for 2 consecutive days), each programmed to occur randomly with respect to time and each other with the restriction that the CS and US could not overlap.
Post-Acquisition Test
All animals received behavioral testing identical to the pretraining (baseline) test measurement for phototaxis 24 h after the second conditioning session. Animals that did not initiate locomotion in the presence of the CS within 20 min during the posttest received a maximum latency score. Assessment of conditioning was determined by computing suppression ratios that compared posttraining phototactic behavior with pretraining test scores. The ratio is expressedas(A/A + B) where A represents pretraining scores and B represents posttraining scores. Conditioned animals exhibited behavioral suppression that was similar in magnitude to previous reports (Crow and Alkon 1978; Crow and Offenbach 1983). After postacquisition testing all animals were coded so that the collection of electrophysiological data was conducted using completely blind experimental procedures.
Intracellular Recordings
Intracellular recordings from identified type I interneurons and VP1 pedal neurons were collected from isolated nervous systems. The type I interneurons were localized to a region of the cerebropleural ganglion as noted in previous publications (Akaike and Alkon 1980; Crow and Tian 2000, 2002a). Surgical desheathing of a small area of the cerebropleural and pedal ganglion was conducted to expose the cell bodies of type I interneurons and pedal neurons. Type I interneurons were identified based upon soma size, cell layer, location in the cerebropleural ganglion, and light-elicited complex PSPs as previously reported (Crow and Tian 2000, 2002a). Pedal neurons were identified by size, position along the anterior-ventral edge of the pedal ganglia, and electrophysiological responses to light and extrinsic current stimulation of pedal nerves and interneurons.
The partially desheathed circumesophageal nervous systems were pinned to a silicone elastomer (Sylgard; Dow Chemical) stage in a recording chamber filled with ASW of the following composition (mM): 460 NaCl, 10 KCl, 10 CaCl2, 55 MgCl2, buffered with 10 mM HEPES and brought to pH 7.46 with dilute NaOH. The ASW in the recording chamber was monitored by a thermistor and held at 15 ±0.5°C. CS illumination of the eyes was provided by a tungsten halogen incandescent lamp attached to a fiber optic bundle mounted underneath the recording chamber. For simultaneous recordings, identified pairs of type I interneurons and pedal neuron VP1 were impaled with microelectrodes filled with 4m KAc and connected to the two headstages of an Axoclamp 2A (Axon Instruments). Electrode resistances varied between 60 and 90 MΩ. Digitized data were analyzed and plotted using Spike 2 Software (Cambridge Electronics Design). Spikes and PSPs evoked by the CS, and elicited by 1-min and 5-min presentations of light were collected from conditioned animals and pseudorandom controls. IPSPs recorded in VP1 were evoked by 2-sec and 5-sec extrinsic depolarizing current pulses applied to type Ie interneurons. Baseline activity for spikes and IPSPs in VP1 pedal neurons was collected during the same time period immediately preceding light or current stimulation. Excitability of type Ie interneurons was assessed with 0.1 nA, 0.2 nA, and 0.3 nA depolarizing 2-sec extrinsic current pulses. Effects involving more than two groups were assessed with an ANOVA followed by posthoc comparisons (Tukey tests). Two group comparisons involved t-tests for independent groups.
Acknowledgments
We thank Diana Parker for assistance with the manuscript. This research was supported by National Institutes of Health Grant MH-58698.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.58603.
References
- Akaike, T. and Alkon, D.L. 1980. Sensory convergence on central visual neurons in Hermissenda. J. Neurophysiol. 44: 501–513. [DOI] [PubMed] [Google Scholar]
- Alkon, D.L. 1973a. Neural organization of a molluscan visual system. J. Gen. Physiol. 61: 444–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon, D.L. 1973b. Intersensory interactions in Hermissenda. J. Gen. Physiol. 62: 185–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon, D.L. and Fuortes, M.G.F. 1972. Responses of photoreceptors in Hermissenda. J. Gen. Physiol. 60: 631–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon, D.L., Akaike, T., and Harrigan, J. 1978. Interaction of chemosensory, visual and statocyst pathways in Hermissenda crassicornis. J. Gen. Physiol. 71: 177–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon, D.L., Lederhendler, I., and Shoukimas, J.J. 1982. Primary changes of membrane currents during retention of associative learning. Science 215: 693–695. [DOI] [PubMed] [Google Scholar]
- Alkon, D.L., Sakakibara, M., Forman, R., Harrigan, J., Lederhendler, I., and Farley, J. 1985. Reduction of two voltage-dependent K+ currents mediates retention of a learned association. Behav. Neurol. Biol. 44: 278–300. [DOI] [PubMed] [Google Scholar]
- Benjamin, P.R., Stanas, K., and Kemones, G. 2000. A systems approach to the cellular analysis of associative learning in the Lymnaea. Learn. Mem. 7: 124–131. [DOI] [PubMed] [Google Scholar]
- Burrell, B.D., Sahley, C.L, and Muller, K.J. 2001. Nonassociative learning and serotonin induce similar bidirectional change in excitability of a neuron critical for learning in the medicinal leech. J. Neurosci. 24: 1401–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary, L.J., Byrne, J.H., and Frost, W.N. 1995. Role of interneurons in defensive withdrawal reflexes in Aplysia. Learn. Mem. 2: 133–151. [DOI] [PubMed] [Google Scholar]
- Collin, C.C., Ikeno, H., Harrigan, J.F., Lederhendler, I., and Alkon, D.L. 1988. Sequential modifications of membrane currents with classical conditioning. Biophys. J. 54: 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow, T. and Alkon, D.L. 1978. Retention of an associative behavioral change in Hermissenda. Science 201: 1239–1241. [DOI] [PubMed] [Google Scholar]
- Crow, T. and Alkon, D.L. 1980. Associative behavioral modification in Hermissenda: Cellular correlates. Science 209: 412–414. [DOI] [PubMed] [Google Scholar]
- Crow, T. and Offenbach, N. 1983. Modification of the initiation of locomotion in Hermissenda: Behavioral analysis. Brain Res. 271: 301–310. [DOI] [PubMed] [Google Scholar]
- Crow, T. and Tian, L.-M. 2000. Monosynaptic connections between identified A and B photoreceptors and interneurons in Hermissenda: Evidence for labeled-lines. J. Neurophysiol. 84: 367–375. [DOI] [PubMed] [Google Scholar]
- Crow, T. and Tian, L.-M. 2002a. Morphological characteristics and central projections of two types of interneurons in the visual pathway of Hermissenda. J. Neurophysiol. 87: 322–332. [DOI] [PubMed] [Google Scholar]
- Crow, T. and Tian, L.-M. 2002b. Facilitation of monosynaptic and complex PSPs in type I interneurons of conditioned Hermissenda. J. Neurosci. 22: 7818–7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow, T. and Tian, L.-M. 2003. Interneuronal projections to identified cilia-activating pedal neurons in Hermissenda. J. Neurophysiol. (in press) [DOI] [PubMed]
- Crow, T., Heldman, E., Hacopian, V., Enos, R., and Alkon, D.L. 1979. Ultrastructure of photoreceptors in the eye of Hermissenda labeled with intracellular injections of horseradish peroxidase. J. Neurocyt. 8: 181–195. [DOI] [PubMed] [Google Scholar]
- Disterhoft, J.F., Coulter, D.A., and Alkon, D.L. 1986. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc. Natl. Acad. Sci. 83: 2733–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermentrout, B., Wang, J.W., Flores, J., and Gelperin, A. 2001. Model for olfactory discrimination and learning in Limax procerebrum incorporating oscillatory dynamics and wave propagation. J. Neurophysiol. 85: 1444–1452. [DOI] [PubMed] [Google Scholar]
- Farley, J. and Alkon, D.L. 1982. Associative neural and behavioral change in Hermissenda: Consequences of nervous system orientation for light- and pairing-specificity. J. Neurophysiol. 48: 785–807. [DOI] [PubMed] [Google Scholar]
- Frysztak, R.J. and Crow, T. 1993. Differential expression of correlates of classical conditioning in identified medial and lateral type A photoreceptors of Hermissenda. J. Neurosci. 13: 2889–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frysztak, R.J, and Crow, T. 1994. Enhancement of type B and A photoreceptor inhibitory synaptic connections in conditioned Hermissenda. J. Neurosci. 14: 1245–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frysztak, R.J. and Crow, T. 1997. Synaptic enhancement and enhanced excitability in presynaptic and postsynaptic neurons in the conditioned stimulus pathway of Hermissenda. J. Neurosci. 17: 4426–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi, C.L. and Matzel, L.D. 2000. Modulation of presynaptic action potential kinetics underlies synaptic facilitation of type B photoreceptors after associative conditioning in Hermissenda. J. Neurosci. 20: 2022–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelperin, A. and Flores, J. 1997. Vital staining from dye-coated microprobes identifies new olfactory interneurons for optical and electrical recording. J. Neurosci. Meth. 72: 97–108. [DOI] [PubMed] [Google Scholar]
- Goh, Y. and Alkon, D.L. 1984. Sensory, interneuronal, and motor interactions within Hermissenda visual pathway. J. Neurophysiol. 52: 156–169. [DOI] [PubMed] [Google Scholar]
- Goh, Y., Lederhendler, J., and Alkon, D.L. 1985. Input and output changes of an identified neural pathway are correlated with associative learning in Hermissenda. J. Neurosci. 5: 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson, T.M. and Crow, T. 1991. Characterization of four light responsive putative motor neurons in the pedal ganglia of Hermissenda crassicornis. Brain Res. 557: 255–264. [DOI] [PubMed] [Google Scholar]
- Hodgson, T.M. and Crow, T. 1992. Cellular correlates of classical conditioning in identified light responsive pedal neurons of Hermissenda crassicornis. Brain Res. 570: 267–271. [DOI] [PubMed] [Google Scholar]
- Jerussi, T.P. and Alkon, D.L. 1981. Ocular and extraocular responses of identifiable neurons in pedal ganglia of Hermissenda crassicornis. J. Neurophysiol. 46: 659–671. [DOI] [PubMed] [Google Scholar]
- Kleinfeld, D., Delaney, K.R., Fee, M.S., Flores, J.A., Tank, D.W., and Gelperin, A. 1994. Dynamics of propagating waves in the olfactory network of a terrestrial mollusk: An electrical and optical study. J. Neurophysiol. 72: 1402–1419. [DOI] [PubMed] [Google Scholar]
- Kovac, M.P., Matera, E.M., Volk, P.J., and Davis, W.J. 1986. Food avoidance learning is accompanied by synaptic attenuation in identified interneurons controlling feeding behavior in Pleurobranchaea. J. Neurophysiol. 56: 891–905. [DOI] [PubMed] [Google Scholar]
- Lederhendler, I.I., Gart, S., and Alkon, D.L. 1986. Classical conditioning of Hermissenda: Origin of a new response. J. Neurosci. 6: 1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer, J.R., Thompson, L.T., and Disterhoft, J.F. 1996. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J. Neurosci. 16: 5536–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, W.G. and Farley, J. 1987. Motor correlates of phototaxis and associative learning. Brain Res. Bull. 19: 174–189. [DOI] [PubMed] [Google Scholar]
- Sahley, C.L. and Crow, T. 1998. Invertebrate learning: Current perspectives. In Learning and Memory (eds. J.L. Martinez Jr., and R.P. Kesner), pp. 177–209. Academic Press, New York, NY.
- Schreurs, B.G. 1989. Classical conditioning of model systems: A behavioral review. Psychobiol. 17: 145–155. [Google Scholar]
- Thompson, L.T., Moyer, J.R., and Disterhoft, J.F. 1996. Transient changes in excitability of rabbit CA3 neurons with a time-course appropriate to support memory consolidation. J. Neurophysiol. 70: 1210–1220. [DOI] [PubMed] [Google Scholar]
- West, A., Barnes, E.S., and Alkon, D.L. 1982. Primary changes of voltage responses during retention of associative learning. J. Neurophysiol. 48: 1243–1255. [DOI] [PubMed] [Google Scholar]
- Woody, C.D. and Engel Jr., J. 1972. Changes in unit activity and thresholds to electrical stimulation of coronal-pericruciate cortex of cat with classical conditioning of different facial movements. J. Neurophysiol. 35: 230–241. [DOI] [PubMed] [Google Scholar]
- Woody, C.D., Knispel, J.D., Crow, T.J., and Black-Cleworth, P.A. 1976. Activity and excitability to electrical current of cortical auditory receptive neurons of awake cats as affected by stimulus association. J. Neurophysiol. 39: 1045–1061. [DOI] [PubMed] [Google Scholar]