Abstract
Rapidly identifying known individuals is an essential skill in human society. To elucidate the neural basis of this skill, we monitored brain activity while experimental participants demonstrated their ability to recognize people on the basis of viewing their faces. Each participant first memorized the faces of 20 individuals who were not known to the participants in advance. Each face was presented along with a voice simulating the individual speaking their name and a biographical fact. Following this learning procedure, the associated verbal information could be recalled accurately in response to each face. These learned faces were subsequently viewed together with new faces in a memory task. Subjects made a yes–no recognition decision in response to each face while also covertly retrieving the person-specific information associated with each learned face. Brain activity that accompanied this retrieval of person-specific information was contrasted to that when new faces were processed. Functional magnetic resonance imaging in 10 participants showed that several brain regions were activated during blocks of learned faces, including left hippocampus, left middle temporal gyrus, left insula, and bilateral cerebellum. Recordings of event-related brain potentials in 10 other participants tracked the time course of face processing and showed that learned faces engaged neural activity responsible for person recognition 300–600 msec after face onset. Collectively, these results suggest that the visual input of a recently learned face can rapidly trigger retrieval of associated person-specific information through reactivation of distributed cortical networks linked via hippocampal connections.
Memory for people requires linking together diverse sorts of information, including a person's name, physical appearance, personal characteristics, and relevant interpersonal interactions. A brief glimpse of a face can provoke the rapid retrieval of a wealth of stored information pertaining to that person. However, the computational steps required for this process of person recognition are not well understood.
How is person-specific information represented in the brain, and what happens to allow that information to be retrieved? When retrieval is cued by facial input, structural analysis in the visual system is the first step. Investigations of face processing using single-unit physiology (e.g., Sugase et al. 1999), scalp electrophysiology (e.g., Bentin et al. 1996), intracranial electrophysiology (e.g., Allison et al., 1994), magnetoencephalography (e.g., Sams et al. 1997), and neuroimaging (e.g., Kanwisher et al. 1997), converge to suggest that structural processing of a face is mediated by a large portion of the ventral stream from primary visual cortex to inferotemporal cortex. Some portion of this processing may also contribute to person recognition, given that selective face recognition deficits tend to result from damage to posterior temporal regions, particularly on the right (Damasio et al. 1990; Carlesimo and Caltagirone 1995; De Renzi 1997). For such patients with prosopagnosia, the face of a known person does not lead to successful retrieval of previously associated biographical information.
Nonetheless, person recognition requires more than the structural analysis of a face. Further, it is mediated not by a single cortical region, but rather by virtue of links established between networks representing the visual information that defines an individual's physiognomy and networks representing the multidimensional information that uniquely characterizes that individual. This linking of information represented in multiple neocortical regions is a quintessential feature of declarative memory (Paller 1997, 2002), as disrupted in cases of circumscribed amnesia due to medial temporal brain damage.
Prior neuroimaging studies have examined the neural basis of person recognition by contrasting activity during viewing of famous faces with activity elicited by unfamiliar faces (e.g., Sergent et al. 1992; Kapur et al. 1995; Andreasen et al. 1996; Haxby et al. 1996). Unfortunately, interpreting results from studies of memory for well-known people can be problematic because it is difficult to match facial qualities between known and unknown individuals and also to know exactly what information was retrieved. In contrast, physical features and person-specific information can be systematically manipulated when pre-experimentally unfamiliar faces are used. Most importantly for present purposes, nonfamous faces can be assigned randomly to conditions in a memory test and counterbalanced across subjects.
In prior investigations of person recognition conducted in this manner, we used a procedure for simulating the experience of meeting 20 people (Paller et al. 1999, 2000). Experimental participants accurately discriminated these learned faces from new faces, and concurrently recorded event-related potentials (ERPs) differed reliably between learned and new faces. A neural signature of the recollection of person-specific information was identified, but it was not possible to precisely localize the relevant brain regions.
Accordingly, in the present study we investigated person recognition using both whole-brain functional magnetic resonance imaging (fMRI) and ERP recordings, in an attempt to more fully characterize the neural dynamics of person recognition. In the initial phase of the experiment, participants learned the faces of 20 previously unfamiliar individuals along with associations between each face and a distinct name, voice, and biographical fact. Next, in the test phase, fMRI or ERP brain responses were monitored while participants performed a recognition memory task for the learned faces, as well as a gender-classification task that placed minimal demands on memory retrieval processing. The memory task was performed with different proportions of learned and new faces in the heavy-retrieval and light-retrieval conditions, as shown in Figure 1. The gender-classification task was performed with new faces. All new faces were unique (i.e., each new face used in the experiment was presented in the memory task or in the gender task on only one occasion). Subsequently, a face/nonface discrimination task was used to identify face-responsive brain regions. The contrast between memory and gender conditions was used to identify neural correlates of face-cued retrieval during a recognition test versus face processing in the absence of memorial requirements, whereas the contrast between heavy-retrieval and light-retrieval conditions was used to isolate neural correlates of successfully remembering person-specific information.
Figure 1.
Example of a block of face presentations and the defining features of each experimental condition. Each block of faces started with a 300-msec task cue, followed by an 1800-msec fixation cross and 10 face trials (300-msec face and 2100-msec fixation cross). Faces were presented briefly to discourage eye movements and to maximize time-locking of relevant cognitive processes. There were 2 runs of 18 blocks (26.1 sec per block, corresponding to 6 whole-brain scans). The gender task was performed in every third block and the memory task in all other blocks, alternating between heavy-retrieval blocks and light-retrieval blocks. In the memory task, subjects made a recognition judgment for each face and were instructed to covertly retrieve the learned biographical information when cued by the associated face.
RESULTS
Task Performance
Response accuracy was nearly perfect in both the memory task and the gender task in all 20 subjects. Recognition decisions were 95% correct (SE = 0.7) with a mean response latency of 769 msec (SE = 20). Neither accuracy nor latency differed significantly between learned and new faces. Gender decisions were 97% correct (SE = 0.5) with a mean response latency of 678 msec (SE = 19).
In the final memory test, given after neural data acquisition was concluded, subjects recognized 96% of the learned faces (SE = 1.7) and made false positive responses for 3% of the new faces (SE = 1.1). Recall performance averaged 77% correct for names (SE = 5.7) and 93% correct for the other biographical information (SE = 2.4).
fMRI Comparisons Between Heavy and Light Retrieval
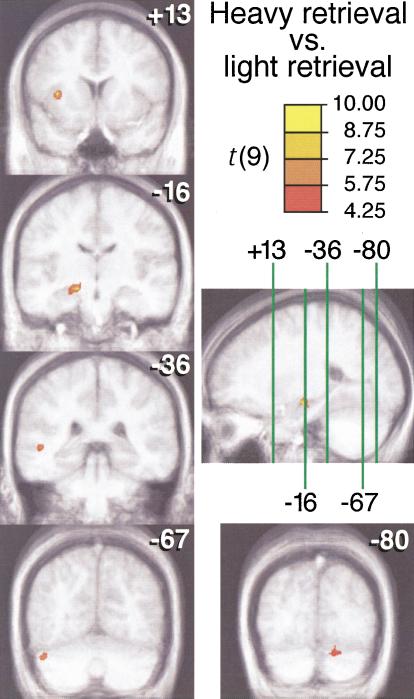
The contrast between the two conditions of memory testing held general task requirements constant while varying the relative frequency with which person-specific information was successfully retrieved. Brain regions differentially activated in these two conditions, heavy retrieval and light retrieval, are shown in Figure 2 and listed in Table 1. In particular, a large medial temporal region in the left hemisphere, including the left hippocampus, was activated when learned faces were presented such that corresponding biographical information was retrieved. Left middle temporal gyrus, left insula, and bilateral cerebellum were also activated in this contrast. No regions showed significantly greater activation for light retrieval than for heavy retrieval.
Figure 2.
Brain activations from the heavy-retrieval vs. light-retrieval contrast. Activation maps were generated according to an analysis of signal change using a threshold of t >4.25 (as shown on color scale) with a cluster threshold of 500 mm3 and connectivity radius of 2.5 mm. Activations are shown superimposed on cross-subject average structural MRI scans. Coronal images are labeled according to Y Talairach coordinate, and these slice locations are shown on a sagital image through the left medial temporal region at the level of X = –23. For each cluster, activity was greater for heavy retrieval than for light retrieval.
Table 1.
Significant Activations for the Heavy Retrieval > Light Retrieval Contrast
|
Talairach coordinates
|
|||||
|---|---|---|---|---|---|
| Brain region (Brodmann area) | Volume in mm3 | X | Y | Z | Mean t-value |
| Left insula | 609 | -33 | 13 | 4 | 5.61 |
| Left medial temporal region, including left hippocampus | 1672 | -14 | -18 | -11 | 5.31 |
| Left middle temporal gyrus (BA 21/22) | 703 | -50 | -36 | 1 | 5.27 |
| Left cerebellum | 500 | -46 | -67 | -29 | 5.26 |
| Right cerebellum | 750 | 16 | -80 | -26 | 4.80 |
Note. Activations listed in anterior-to-posterior order.
fMRI Comparisons Between Memory and Gender Tasks
The contrast between tasks allowed us to identify regions differentially active during episodic retrieval attempts versus gender categorization while controlling general perceptual and response factors. Brain regions differentially activated in the two conditions are listed in Table 2. Three right-lateralized regions—right precuneus, right dorsolateral prefrontal cortex, and right insula— were activated more during memory judgments than during gender judgments, whereas left superior prefrontal and bilateral parietal regions exhibited the opposite pattern. The robust right dorsolateral prefrontal activation observed in this contrast is consistent with suggestions that this region is involved in the initiation of a retrieval attempt (Buckner and Wheeler 2001), perhaps by establishing an episodic retrieval mode (Lepage et al. 1998). Alternatively, right prefrontal regions may function to implement retrieval strategies for accessing relevant information using simple heuristics (Nolde et al. 1998) or coarse-coding strategies (Beeman 1998). Another possible reason for the right-sided nature of this frontal activation could be related to right-hemisphere dominance in processing facial stimuli. However, direct investigations of material-specific laterality effects have generally reported right-lateralized activation in prefrontal regions posterior to the activations reported here, along the inferior frontal gyrus and precentral sulcus (Kelley et al. 1998; Wagner 1999; Golby et al. 2001). Furthermore, in light of the fact that faces were presented during both the memory task and the gender task, the right dorsolateral prefrontal activation in the contrast between the two tasks is more likely related to memory retrieval than simply to perceptual processing of faces.
Table 2.
Significant Activations in Comparing Memory and Gender Tasks
|
Talairach coordinates
|
|||||
|---|---|---|---|---|---|
| Brain region (Brodmann area) | Volume in mm3 | X | Y | Z | Mean t-value |
| Memory > Gender | |||||
| Right middle frontal gyrus (BA 46) | 938 | 39 | 28 | 26 | 5.50 |
| Right anterior insula | 2391 | 32 | 23 | -1 | 5.96 |
| Midbrain/hypothalamus | 688 | 1 | -25 | -13 | 5.42 |
| Right inferior parietal lobule (BA 7/40) | 9781 | 36 | -59 | 46 | 5.26 |
| and precuneus (BA 7/31) | 17 | -66 | 35 | ||
| Left cerebellum | 1766 | -10 | -83 | -29 | 5.56 |
| Left calcarine sulcus (BA 17) | 969 | 1 | -83 | 8 | 5.14 |
| Left cuneus (BA 18) | 562 | -24 | -92 | -1 | 4.98 |
| Gender > Memory | |||||
| Left superior frontal gyrus (BA 9) | 1156 | -9 | 53 | 21 | 5.25 |
| Right mid-cingulate gyrus (BA 24) | 844 | 1 | -2 | 45 | 5.38 |
| Left superior frontal gyrus (BA 6) | 500 | -22 | -13 | 70 | 4.99 |
| Left inferior parietal lobule (BA 40) | 516 | -61 | -33 | 38 | 5.51 |
| Right inferior parietal lobule (BA 40) | 1672 | 58 | -40 | 36 | 4.75 |
| Right precuneus (BA 7) | 1172 | 11 | -56 | 58 | 5.43 |
Note. Activations listed in anterior-to-posterior order within contrast.
Fusiform fMRI Activations
A face-sensitive region of the fusiform gyrus was identified in the final scanning phase by contrasting face versus scrambled face conditions (e.g., Kanwisher et al. 1997). This region, as identified in the group analysis, was taken as a region of interest to determine whether it was differentially activated across the main conditions. Differences in fusiform activation between memory and gender tasks and between heavy and light retrieval were not statistically significant. However, these null findings do not imply that neural activity in this fusiform region makes no relevant contribution to person recognition. Given current limits on spatial resolution, neuronal activity may increase in some portions of the fusiform, while decreasing in other portions, to yield nonsignificant differences. Also, locations of activity may vary greatly across individuals. Yet, fusiform activations have been associated with the encoding of facial information in other studies (Kuskowski and Pardo 1999). In any event, the robust face/scrambled face activation in the fusiform (location of highest activation at Talairach coordinates, 38, –42, –19) highlights the spatial separation between this fusiform region that is highly responsive to faces and the cerebellar regions activated in the heavy/light retrieval contrasts.
ERP Comparisons Between Learned Faces and New Faces
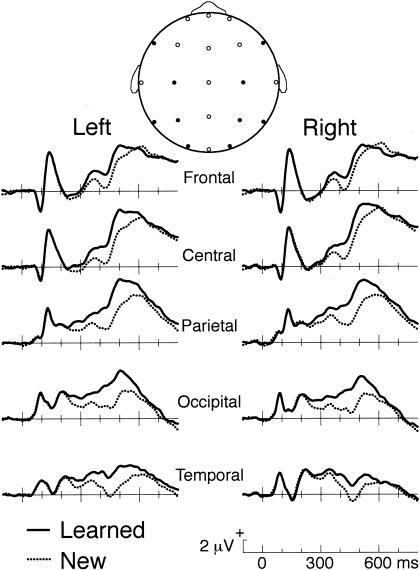
As shown in Figure 3, differential responses for learned versus new faces took the form of an enhanced positive response maximal between 400–500 msec. ERPs were measured at all scalp locations over 100-msec intervals and found to differ significantly between conditions from 300–600 msec [F(1,9) ≥ 8.5, P ≤ .017]. The learned-new ERP difference over this interval was reliably larger at left-relative to right-hemisphere locations [F(1,9) = 5.4, P = .045] and was maximal at the midline parietal location (3.0 μV, SE = 0.7). When tested separately for the homologous left/right locations depicted in Figure 3, the learned-new difference was reliably larger on the left side for two electrode pairs, lateral frontal [F(1,9) = 16.8, P = .003] and posterior temporal [F(1,9) = 5.6, P = .043].
Figure 3.
Brain potentials for learned faces and new faces. Responses are shown for trials during the memory task in which behavioral responses were correct. Recordings were from scalp locations from the International 10-20 System. These electrode locations are shown as filled circles in a schematic representation of a head viewed from above (F7/F8, C3/C4, P3/P4, O1/O2, and T5/T6); other electrode locations in which EEG data were recorded are shown as open circles.
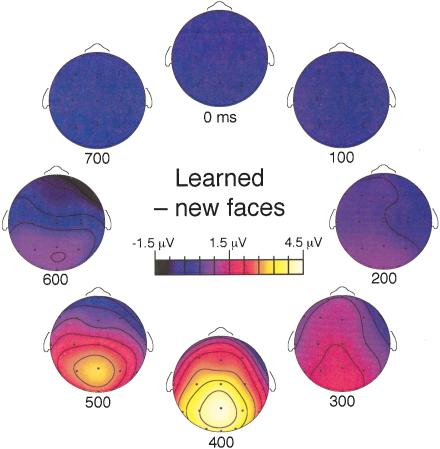
Maps of the scalp distribution of differential ERP activity for learned versus new faces were created for consecutive 100-msec windows (Fig. 4). Both frontal and posterior regions of brain activity can be observed. The frontal portion of the learned-new difference was clear from 300–500 msec. In contrast, the effect was apparent at posterior scalp locations for a longer time, extending to the interval from 600–700 msec.
Figure 4.
Temporal progression of topographic maps for electrophysiological differences between learned and new faces. Maps were created using a spline interpolation on the basis of mean amplitude measurements from each of 21 electrode locations (shown as open circles on schematic representations of a head viewed from above) over consecutive 100-msec intervals beginning at the times indicated below each map.
DISCUSSION
Our findings help characterize brain events responsible for person recognition in both spatial and temporal dimensions. A distributed network linked by the left hippocampus appeared to support retrieval of person-specific information. Complementary electrophysiological results demonstrated that differential processing of learned and new faces occurred from 300–600 msec after face onset.
Person recognition resulted when a sensory image of a face made contact with stored information about the person depicted, a memory process referred to generally as ecphory (Tulving 1983). However, subjects did not overtly produce the person-specific information they had memorized when a learned face appeared in the test phase. Instead, manual responses indicated successful discrimination between learned and new faces. This absence of overt recall was advantageous because it eliminated interpretive difficulties due to differential behavioral responses.
How can we be sure that learned faces did indeed evoke the covert retrieval of biographical information? Although it is unclear whether physical characteristics of the voices presented in the study phase were recalled when the associated face appeared, several considerations support the inference that recognition performance wasn't supported merely by context-free face familiarity without biographical recall. First, recall results confirmed that the person-specific information had been effectively committed to memory. Second, subjects were instructed to bring this information to mind during the memory task. Most persuasively, real-time electrophysiological data implicated recollective processing, on the basis of the following reasoning.
ERP differences between learned and new faces in the present experiment bore a strong resemblance to those in two prior studies (Paller et al. 1999, 2000). This correspondence suggests that similar memory processing occurred across studies, even though designs differed in whether stimuli were blocked or randomized, whether overt recognition responses were required, and whether learning occurred 1 d or only a few minutes in advance. A convincing association between electrophysiological effects and recollection, as opposed to nonconscious memory, was found by Paller et al. (1999) on the basis of the fact that the two critical conditions differed in recognition but were matched in perceptual priming, and in physical stimulus characteristics. Subsequent comparisons between faces with and without spoken vignettes (Paller et al. 2000) showed qualitatively different ERP patterns at retrieval, suggesting that posterior ERPs were related to facial memory and anterior ERPs to nonfacial memory (e.g., biographical retrieval). Finally, in a recent study focusing on the experience of pure familiarity, brain potentials recorded when subjects viewed a face that provoked retrieval of contextual information associated with that face resembled those in the present experiment (G. Yovel and K.A. Paller, in prep.). Thus, we infer that differential neural activity for learned versus new faces in the present experiment reflects recollective processing of both facial and other biographical information.
Neuroanatomical Networks for Person Recognition
Facial information was analyzed in both gender and memory tasks, but with different objectives—gender-specific cues emphasized in one case, information that stimulated retrieval in the other, including retrieval of the spatiotemporal context of the initial episode of viewing the face and of associated factual information. Both tasks thus engaged many cortical visual areas, including a portion of the fusiform gyrus implicated previously in face processing. Activation of this area does not index face recognition, given that it is relatively unaffected by familiarity (Gorno Tempini et al. 1998; Dubois et al. 1999) or inversion (Aguirre et al. 1999; Haxby et al. 1999), although an adjacent midfusiform area was associated with recognizing famous faces (George et al. 1999). Although the fusiform face area may be critical for the structural analysis of faces, it is probably not the storehouse of the information used when we remember the people we know.
Amnesic patients typically have difficulty remembering exactly the sort of information memorized by participants in our experiment. Storing these memories requires that information represented in multiple cortical regions be linked together, and the hippocampus is thought to play a key role in this process (Rempel-Clower et al. 1996; Mayes and Downes 1997; Moscovitch and Nadel 1998). In addition, hippocampal activity has been associated with encoding face-name associations (Sperling et al. 2001). Thus, a reasonable prediction is that learned faces in the present experiment would elicit hippocampal activity related to retrieval processing (e.g., Stark and Squire 2000). One might even argue that remembering a face without any biographical information would depend on the hippocampus, to the extent that memory is supported by associations between the face and contextual information. On the other hand, greater medial temporal activation for novel relative to familiar stimuli was observed in other studies (Tulving et al. 1994; Stern et al. 1996). Nevertheless, many recent studies suggest that stimulus novelty effects are robust in the parahippocampal gyrus, but relatively less consistent in the hippocampus (Martin et al. 1997; Constable et al. 2000; Zeinch et al. 2000; Ranganath and D'Esposito 2001; Reber et al. 2002). The literature on memory-related activations within the hippocampus proper has revealed a complex mix of findings during encoding and retrieval (Lepage et al. 1998; Schacter and Wagner 1999). Nonetheless, our finding of robust left hippocampal activity associated with successful retrieval of person-specific information is consistent with the view that hippocampal–cortical interactions during retrieval support the gradual strengthening of distributed cortical representations characteristic of declarative memories (Paller 2002).
Hippocampal activation occurred not in isolation, but together with activation of a nearby left temporal region, the left insula, and the cerebellum bilaterally. Results from several sources also implicate temporal neocortex in person recognition. Memory impairments in patients with focal retrograde amnesia (Kapur 1993; Markowitsch 1995) and semantic dementia (Graham et al. 1999) suggest that knowledge about people may be stored in temporal neocortex in the form of thematic frameworks or coherence ensembles (Hodges and McCarthy 1995; Paller 1997). A selective disruption of face recognition and person-specific semantic knowledge has been described in patients with right anterior temporal damage (Ellis et al. 1989; Evans et al. 1995; Kitchener and Hodges 1999). Lesion evidence has also implicated left temporal regions in the retrieval of people's names (e.g., Semenza et al. 1995; Tsukiura et al. 2002).
Neuroimaging results have also underscored the importance of temporal neocortex for person recognition, along with other regions. Some investigators have suggested that associating faces with other information, such as a name, is dependent upon left supramarginal gyrus (Campanella et al. 2001) and retrosplenial cortex/posterior cingulate (Shah et al. 2001). Tsukiura et al. (2002) concluded that anterior temporal cortex is critical for this retrieval, particularly in the left hemisphere, with changes over time involving cortical reorganization. Moreover, fMRI activations in bilateral prefrontal, lateral-temporal, and medial-temporal regions were elicited by famous faces compared with new faces (Leveroni et al. 2000). Retrieving semantic information pertaining to famous people has also been associated with activity in left lateral temporal cortex (Gorno Tempini et al. 1998) and in the temporal poles (Damasio et al. 1996). For famous faces, of course, the nature and amount of learned person-specific information is difficult to quantify, given the lack of control over learning. Nevertheless, the extant evidence taken together with the present results suggest that conjoint activation of medial and anterolateral temporal regions plays a central role in remembering people successfully.
Temporal Dynamics of Person Recognition
Differences between brain potentials to learned versus new faces first appeared at ∼225 msec and were statistically reliable from 300–600 msec after face onset, well before behavioral recognition decisions. This differential electrophysiological response included a left-frontal effect at 300–500 msec and an overlapping effect with a left posterior topography, higher amplitude, and longer duration. Earlier potentials such as the face-sensitive N170 (Bentin et al. 1996; Puce et al. 1999) did not differ between conditions. Although complete integration of electrophysiological and hemodynamic data would require an appropriate model of intracranial current flows (Mangun et al. 1998; Rugg 1998), such modeling is seldom satisfactory when large numbers of distributed generators are active, as appears to be the case with person recognition. A suitable strategy for successful ERP-fMRI data merging would thus be for future studies to fractionate person recognition by isolating distinct subcomponent processes.
Nonetheless, our ERP results provide some insight into the temporal dynamics of neural activity responsible for person recognition. Although the fMRI analysis was based on a blocked design, ERP findings showed that differential electrophysiological responses were elicited by single-face presentations within each block. Whereas fMRI activations may have included neural activity differentially associated with state changes in heavy versus light retrieval blocks (Düzel et al. 1999; Donaldson et al. 2001), ERP responses included only neural activity time-locked to face presentation. The relative timing of the frontal and posterior ERPs suggest that person-specific information may have been retrieved through rapid interactions within 500 msec between frontal and temporal cortical networks linked together via connections between these networks and hippocampal networks.
Person Recognition and Memory Retrieval
At a general level, our findings support the idea that cortical regions required for perceiving particular sorts of information also facilitate remembering that information (e.g., Fuster 1995; Mesulam 1998). Consequently, the specific regions involved in memory retrieval vary depending on the information retrieved. Although different information was associated with each of the 20 faces, there was some commonality. In general, successful person recognition can entail retrieval of prior episodes, sensory details pertaining to an individual's face and voice, and biographical information manifest in complex imagery and semantic processing. We propose that the information retrieved in response to each face presentation, including both verbal and nonverbal associations, was stored in a network of frontal and temporal cortical areas, and that combined prefrontal/cerebellar networks played a role in implementing and coordinating retrieval, as well as in evaluating the accuracy of retrieval (Ranganath and Paller 1999; Ranganath et al. 2000, 2003). Left insula may have been activated as verbal information about each person was retrieved and rehearsed, whereas left midtemporal cortex may be associated with the semantic storage of person-specific information (Tsukiura et al. 2002). Left hippocampal activity may reflect specific episodic content (Nadel et al. 2000) and/or the linking function that connects relevant representations in multiple neocortical regions (Paller 2002).
Person recognition is a prime example of the type of remembering that patients with memory disorders find difficult. It depends on the storage of diverse sorts of information in multiple cortical regions. Neuropsychological studies have begun to delineate the distributed network of brain regions responsible for storing and retrieving such memories. Neural correlates of person recognition in neurologically healthy individuals, as identified in the present investigation, can corroborate these findings and help to specify the distinct roles of each region. Further research in this area can thus enhance our understanding of how complex interactions among brain regions can swiftly mediate memory retrieval in response to an appropriate stimulus cue such as the face of a known individual.
MATERIALS AND METHODS
Participants
Right-handed individuals from the university community (13 women and 7 men) were assigned to the fMRI group (n = 10) or the ERP group (n = 10). Mean age was 21.2 years. All were naive to experimental goals and had not previously viewed any of the face stimuli. Informed consent was obtained using a consent form approved by the Northwestern University Institutional Review Board.
Stimuli and Tasks
The same basic procedure with regard to face processing and memory testing was used with both groups of subjects. Grayscale photographs of 448 faces from a 1977 high-school yearbook were presented within a rectangular space (∼6° by 8° visual angle). In the study phase, each of 20 learned faces was paired with a unique, recorded voice presented concurrently. Each voice uttered a name and brief biographical information, such as “I'm Alison and I won the Boston Marathon twice” (see Paller et al. 1999 for a complete list). Subjects made use of these spoken vignettes in order to memorize the faces. Another 20 faces were presented without voices, as in several antecedent experiments (Paller et al. 1999, 2000). The specific faces presented with and without voices were counterbalanced across subjects. One face was displayed every 5 sec, for a 300-msec duration, and the entire set of faces was presented three times, using different random orders. Subjects were instructed to remember only the 20 faces paired with voices and to imagine actually meeting these people. They were advised that they would receive an extra $1 for later recalling the central content of each vignette in a final memory test to be given at the conclusion of the experiment. This learning procedure was administered 1 d in advance and again 10 min in advance of the memory task, when neural data were acquired.
In the memory task, learned faces and new faces, randomly intermixed, were shown without any spoken biographical information. Subjects were instructed in advance on task performance. Right index and middle fingers were used to press response buttons labeled 1 and 2, which were assigned to new and old in the memory task and to male and female in the gender task. Button assignments were repeated over an intercom prior to each experimental run. Importantly, subjects were also encouraged to retrieve the appropriate biographical details when each learned face appeared so as to rehearse this information in preparation for the final memory test. The two experimental runs each included 18 blocks. The gender task was performed every third block and the memory task all other blocks, alternating between heavy-retrieval and light-retrieval blocks. Each block included a task cue and a series of 10 faces, as shown in Figure 1.
Face/nonface processing was contrasted in two additional runs. Subjects viewed faces and a scrambled face, which was created by rearranging small parts of a face such that no facial features were identifiable. Faces in run 3 were all new faces and in run 4 faces presented previously in the study phase without voices. Results from runs 3 and 4 were combined, given minimal differences between runs. Subjects pressed a button after each stimulus, button 1 for faces and button 2 for the scrambled face. Alternating 26.1-sec blocks included either 12 faces or 12 repeated presentations of the scrambled face (300-msec stimulus plus 1875-msec fixation cross).
Following fMRI or ERP data acquisition, the final memory test was administered using 100 faces on 5 pages (40 from the study phase and 60 faces not viewed previously). Instructions were to attempt to label each of the 20 learned faces with the corresponding name and biographical information.
fMRI Procedures
Scanning was conducted using a Siemens Vision 1.5-T magnet and head coil. The subject's head was positioned in the isocenter of the magnet and secured comfortably using padding and a vacuum-immobilizer device. Stimuli were projected onto a rear-projection screen and viewed though a mirror. A susceptibility weighted single-shot echoplanar sequence (TR 4.35 sec, TE 40 msec, flip angle 90°, FOV 220 mm, 64 × 64 matrix, 32 4-mm thick slices, resolution 3.44 × 3.44 mm) was used to obtain functional images. The initial four volumes from each run were acquired prior to stimulus presentation and were discarded to allow the MR signal to reach steady state. Structural MRI data were acquired using a 3D FLASH sequence (TR 15 msec, TE 5 msec, flip angle 20°, 256 × 256 matrix, 160 1-mm thick slices, resolution .86 × .86 mm).
Block-design analyses were conducted using AFNI software (Cox 1996), including movement correction, three-dimensional coregistration through time, transformation to a standard stereo-taxic space (MNI-305), and spatial smoothing (7 mm FWHM). Within each run, voxels were eliminated if signal magnitude changed more than 10% between samples, or if the mean signal level was below a threshold. Data were then submitted to a random-effects analysis on the basis of a modified general linear model. Contrasts between memory and gender tasks, between heavy and light retrieval blocks within the memory task, and between face and nonface blocks for the final two experimental runs were based on a boxcar function convolved with an idealized estimate of the hemodynamic response. Regions deemed to exhibit a significant difference were those in which each voxel exhibited a reliable change in activity across participants, t(9) > 4.25, in a 500-mm3 or larger region (i.e., at least 32 voxels in the normalized space at 2.5-mm3 resolution). Monte Carlo simulations using normally distributed noise indicated <5% false positives per experiment with this statistical threshold.
ERP Recording Procedures
The same procedure as described above was followed, except that subjects viewed stimuli on a video monitor while seated in a comfortable chair inside a sound-attenuating chamber. Electro-encephalographic recordings were made from 21 scalp electrodes (bandpass 0.1–100 Hz, sampling rate 250 Hz). The online left mastoid reference was changed digitally to the average of left and right mastoid. Trials contaminated by electro-ocular artifacts were excluded on the basis of recordings from two EOG channels. ERPs measured over various intervals were submitted to repeated-measures ANOVA (α = .05). ERPs were computed separately for learned and new faces across blocks. ERPs were also computed separately for heavy-retrieval and light-retrieval blocks, but these findings were nearly identical to those computed separately by trial type (as expected, given that only 10% of the trials were different). Results reported were based on averaging by trial type using correct trials only.
Acknowledgments
This work was supported by grants from NINDS (NS34639 to K.A.P. and NS30863 to M.M.M.), NIMH (MH58748 to P.J.R.), the McDonnell-Pew Program in Cognitive Neuroscience (D.R.G.), and a NARSAD Young Investigator Award (K.S.L.). C.R. is now at the University of California at Davis, B.G. is now at Stanford University, and K.L. is now at Duke University.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.57403.
References
- Aguirre, G.K., Singh, R., and D'Esposito, M. 1999. Stimulus inversion and the responses of face and object-sensitive cortical areas. Neuroreport 10: 189–194. [DOI] [PubMed] [Google Scholar]
- Allison, T., Ginter, H., McCarthy, G., Nobre, A.C., Puce, A., Luby, M., and Spencer, D.D. 1994. Face recognition in human extrastriate cortex. J. Neurophysiol. 71: 821–825. [DOI] [PubMed] [Google Scholar]
- Andreasen, N.C., O'Leary, D.S., Arndt, S., Cizadlo, T., Hurtig, R., Rezai, K., Watkins, G.L., Ponto, L.B., and Hichwa, R.D. 1996. Neural substrates of facial recognition. J. Neuropsychiat. Clin. Neurosci. 8: 139–146. [DOI] [PubMed] [Google Scholar]
- Beeman, M. 1998. Coarse semantic coding and discourse comprehension. In Right hemisphere language comprehension: Perspectives from cognitive neuroscience (eds. M. Beeman and C. Chiarello), pp. 255–284. Erlbaum, Mahwah, NJ.
- Bentin, S., Allison, T., Puce, A., Perez, E., and McCarthy, G. 1996. Electrophysiological studies of face perception in humans. J. Cogn. Neurosci. 8: 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R.L., and Wheeler, M.E. 2001. The cognitive neuroscience of remembering. Nat. Rev. Neurosci. 2: 624–634. [DOI] [PubMed] [Google Scholar]
- Campanella, S., Joassin, F., Rossion, B., De Volder, A., Bruyer, R., and Crommelinck, M. 2001. Association of the distinct visual representations of faces and names: A PET activation study. NeuroImage 14: 873–882. [DOI] [PubMed] [Google Scholar]
- Carlesimo, G.A. and Caltagirone, C. 1995. Components in the visual processing of known and unknown faces. J. Clin.Exper. Neuropsychol. 17: 691–705. [DOI] [PubMed] [Google Scholar]
- Constable, R.T., Carpentier, A., Pugh, K., Westerveld, M., Oszunar, Y., and Spencer, D.D. 2000. Investigation of the human hippocampal formation using a randomized event-related paradigm and Z-shimmed functional MRI. NeuroImage 12: 55–62. [DOI] [PubMed] [Google Scholar]
- Cox, R.W. 1996. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Damasio, A.R., Tranel, D., and Damasio, H. 1990. Face agnosia and the neural substrates of memory. Annu. Rev. Neurosci. 13: 89–109. [DOI] [PubMed] [Google Scholar]
- Damasio, H., Grabowski, T.J., Tranel, D., Hichwa, R.D., and Damasio, A.R. 1996. A neural basis for lexical retrieval. Nature 380: 499–505. [DOI] [PubMed] [Google Scholar]
- De Renzi, E. 1997. Prosopagnosia. In Behavioral neurology and neuropsychology (eds. T.E. Feinberg and M.J. Farah), pp. 245–255. McGraw-Hill, New York.
- Donaldson, D.I., Petersen, S.E., Ollinger, J.M., and Buckner, R.L. 2001. Dissociating state and item components of recognition memory using fMRI. NeuroImage 13: 129–142. [DOI] [PubMed] [Google Scholar]
- Dubois, S., Rossion, B., Schiltz, C., Bodart, J.M., Michel, C., Bruyer, R., and Crommelinck, M. 1999. Effect of familiarity on the processing of human faces. NeuroImage 9: 278–289. [DOI] [PubMed] [Google Scholar]
- Düzel, E., Cabeza, R., Picton, T.W., Yonelinas, A.P., Scheich, H., Heinze, H.J., and Tulving, E. 1999. Task-related and item-related brain processes of memory retrieval. Proc. Natl. Acad. Sci. 96: 1794–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, A.W., Young, A.W., and Critchley, E.M. 1989. Loss of memory for people following temporal lobe damage. Brain 112: 1469–1483. [DOI] [PubMed] [Google Scholar]
- Evans, J.J., Heggs, A.J., Antoun, N., and Hodges, J.R. 1995. Progressive prosopagnosia associated with selective right temporal lobe atrophy. A new syndrome? Brain 118: 1–13. [DOI] [PubMed] [Google Scholar]
- Fuster, J.M. 1995. Memory in the cerebral cortex. MIT Press, Cambridge, MA.
- George, N., Dolan, R.J., Fink, G.R., Baylis, G.C., Russell, C., and Driver, J. 1999. Contrast polarity and face recognition in the human fusiform gyrus. Nat. Neurosci. 2: 574–580. [DOI] [PubMed] [Google Scholar]
- Golby, A.J., Poldrack, R.A., Brewer, J.B., Spencer, D., Desmond, J.E., Aron, A.P., and Gabrieli, J.D. 2001. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain 124: 1841–1854. [DOI] [PubMed] [Google Scholar]
- Gorno Tempini, M.L., Price, C.J., Josephs, O., Vandenberghe, R., Cappa, S.F., Kapur, N., and Frackowiak, R.S. 1998. The neural systems sustaining face and proper-name processing. Brain 121: 2103–2118. [DOI] [PubMed] [Google Scholar]
- Graham, K.S., Patterson, K., and Hodges, J.R. 1999. Episodic memory: New insights from the study of semantic dementia. Curr. Opin. Neurobiol. 9: 245–250. [DOI] [PubMed] [Google Scholar]
- Haxby, J.V., Ungerleider, L.G., Horwitz, B., Maisog, J.M., Rapoport, S.I., and Grady, C.L. 1996. Face encoding and recognition in the human brain. Proc. Natl. Acad. Sci. 93: 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby, J.V., Ungerleider, L.G., Clark, V.P., Schouten, J.L., Hoffman, E.A., and Martin, A. 1999. The effect of face inversion on activity in human neural systems for face and object perception. Neuron 22: 189–199. [DOI] [PubMed] [Google Scholar]
- Hodges, J.R. and McCarthy, R.A. 1995. Loss of remote memory: A cognitive neuropsychological perspective. Curr. Opin. Neurobiol. 5: 178–183. [DOI] [PubMed] [Google Scholar]
- Kanwisher, N., McDermott, J., and Chun, M.M. 1997. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J. Neurosci. 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur, N. 1993. Focal retrograde amnesia in neurological disease: A critical review. Cortex 29: 217–234. [DOI] [PubMed] [Google Scholar]
- Kapur, N., Friston, K.J., Young, A., Frith, C.D., and Frackowiak, R.S. 1995. Activation of human hippocampal formation during memory for faces: A PET study. Cortex 31: 99–108. [DOI] [PubMed] [Google Scholar]
- Kelley, W.M., Miezin, F.M., McDermott, K.B., Buckner, R.L., Raichle, M.E., Cohen, N.J., Ollinger, J.M., Akbudak, E., Conturo, T.E., Snyder, A.Z., et al. 1998. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron 20: 927–936. [DOI] [PubMed] [Google Scholar]
- Kitchener, E.G. and Hodges, J.R. 1999. Impaired knowledge of famous people and events with intact autobiographical memory in a case of progessive right temporal lobe degeneration: Implications for the organization of remote memory. Cogn. Neuropsychol. 16: 589–607. [Google Scholar]
- Kuskowski, M.A. and Pardo, J.V. 1999. The role of the fusiform gyrus in successful encoding of face stimuli. NeuroImage 9: 599–610. [DOI] [PubMed] [Google Scholar]
- Lepage, M., Habib, R., and Tulving, E. 1998. Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus 8: 313–322. [DOI] [PubMed] [Google Scholar]
- Leveroni, C.L., Seidenberg, M., Mayer, A.R., Mead, L.A., Binder, J.R., and Rao, S.M. 2000. Neural systems underlying the recognition of familiar and newly learned faces. J. Neurosci. 20: 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun, G.R., Hopfinger, J.B., and Heinze, H.-J. 1998. Integrating electrophysiology and neuroimaging in the study of human cognition. Behav. Res. Meth. Instru. Comp. 30: 118–130. [Google Scholar]
- Markowitsch, H.J. 1995. Which brain regions are critically involved in the retrieval of old episodic memory? Brain Res. Rev. 21: 117–127. [DOI] [PubMed] [Google Scholar]
- Martin, A., Wiggs, C.L., and Weisberg, J. 1997. Modulation of human medial temporal lobe activity by form, meaning, and experience. Hippocampus 7: 587–593. [DOI] [PubMed] [Google Scholar]
- Mayes, A.R. and Downes, J.J. 1997. Theories of organic amnesia. Psychology Press, Hove, East Sussex, UK. [DOI] [PubMed]
- Mesulam, M.M. 1998. From sensation to cognition. Brain 121: 1013–1052. [DOI] [PubMed] [Google Scholar]
- Moscovitch, M. and Nadel, L. 1998. Consolidation and the hippocampal complex revisited: In defense of the multiple-trace model. Curr. Opin. Neurobiol. 8: 297–300. [DOI] [PubMed] [Google Scholar]
- Nadel, L., Samsonovich, A., Ryan, L., and Moscovitch, M. 2000. Multiple trace theory of human memory: Computational, neuroimaging, and neuropsychological results. Hippocampus 10: 352–368. [DOI] [PubMed] [Google Scholar]
- Nolde, S.F., Johnson, M.K., and Raye, C.L. 1998. The role of prefrontal cortex during tests of episodic memory. Trends Cogn. Sci. 2: 399–406. [DOI] [PubMed] [Google Scholar]
- Paller, K.A. 1997. Consolidating dispersed neocortical memories: The missing link in amnesia. Memory 5: 73–88. [DOI] [PubMed] [Google Scholar]
- Paller, K.A. 2002. Cross-cortical consolidation as the core defect in amnesia: Prospects for hypothesis-testing with neuropsychology and neuroimaging. In The neuropsychology of memory, 3rd ed. (eds. L.R. Squire and D.L. Schacter), pp. 73–87. Guilford Press, New York.
- Paller, K.A., Bozic, V.S., Ranganath, C., Grabowecky, M., and Yamada, S. 1999. Brain waves following remembered faces index conscious recollection. Cogn. Brain Res. 7: 519–531. [DOI] [PubMed] [Google Scholar]
- Paller, K.A., Gonsalves, B., Grabowecky, M., Bozic, V.S., and Yamada, S. 2000. Electrophysiological correlates of recollecting faces of known and unknown individuals. NeuroImage 11: 98–110. [DOI] [PubMed] [Google Scholar]
- Puce, A., Allison, T., and McCarthy, G. 1999. Electrophysiological studies of human face perception. III: Effects of top-down processing on face-specific potentials. Cerebral Cortex 9: 445–458. [DOI] [PubMed] [Google Scholar]
- Ranganath, C. and D'Esposito, M. 2001. Medial temporal lobe activity associated with active maintenance of novel information. Neuron 31: 865–873. [DOI] [PubMed] [Google Scholar]
- Ranganath, C. and Paller, K.A. 1999. Frontal brain potentials during recognition are modulated by requirements to retrieve perceptual detail. Neuron 22: 605–613. [DOI] [PubMed] [Google Scholar]
- Ranganath, C., Johnson, M.K., and D'Esposito, M. 2000. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J. Neurosci. 20: RC108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath, C., Johnson, M.K., and D'Esposito, M. 2003. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia 41: 378–389. [DOI] [PubMed] [Google Scholar]
- Reber, P.J., Wong, E.C., and Buxton, R.B. 2002. Encoding activity in the medial temporal lobe examined with anatomically constrained fMRI analysis. Hippocampus 12: 363–376. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower, N.L., Zola, S.M., Squire, L.R., and Amaral, D.G. 1996. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J. Neurosci. 16: 5233–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg, M.D. 1998. Convergent approaches to electrophysiological and hemodynamic investigations of memory. Hum. Brain Mapp. 6: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams, M., Hietanen, J.K., Hari, R., Ilmoniemi, R.J., and Lounasmaa, O.V. 1997. Face-specific responses from the human inferior occipito-temporal cortex. Neuroscience 77: 49–55. [DOI] [PubMed] [Google Scholar]
- Schacter, D.L. and Wagner, A.D. 1999. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 9: 7–24. [DOI] [PubMed] [Google Scholar]
- Semenza, C., Mondini, S., and Zettin, M. 1995. The anatomical basis of proper name processing: A critical review. Neurocase 1: 183–188. [Google Scholar]
- Sergent, J., Ohta, S., and MacDonald, B. 1992. Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain 115: 15–36. [DOI] [PubMed] [Google Scholar]
- Shah, N.J., Marshall, J.C., Zafiris, O., Schwab, A., Zilles, K., Markowitsch, H.J., and Fink, G.R. 2001. The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain 124: 804–815. [DOI] [PubMed] [Google Scholar]
- Sperling, R.A., Bates, J.F., Cocchiarella, A.J., Schacter, D.L., Rosen, B.R., and Albert, M.S. 2001. Encoding novel face-name associations: A functional MRI study. Hum. Brain Mapp. 14: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark, C.E. and Squire, L.R. 2000. Functional magnetic resonance imaging (fMRI) activity in the hippocampal region during recognition memory. J. Neurosci. 20: 7776–7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, C.E., Corkin, S., Gonzalez, R.G., Guimaraes, A.R., Baker, J.R., Jennings, P.J., Carr, C.A., Sugiura, R.M., Vedantham, V., and Rosen, B.R. 1996. The hippocampal formation participates in novel picture encoding: Evidence from functional magnetic resonance imaging. Proc. Natl. Acad. Sci. 93: 8660–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugase, Y., Yamane, S., Ueno, S., and Kawano, K. 1999. Global and fine information coded by single neurons in the temporal visual cortex. Nature 400: 869–873. [DOI] [PubMed] [Google Scholar]
- Tsukiura, T., Fujii, T., Fukatsu, R., Otsuki, T., Okuda, J., Umetsu, A., Suzuki, K., Tabuchi, M., Yanagawa, I., Nagasaka, T., et al. 2002. Neural basis of the retrieval of people's names: Evidence from brain-damaged patients and fMRI. J. Cogn. Neurosci. 14: 922–937. [DOI] [PubMed] [Google Scholar]
- Tulving, E. 1983. Elements of episodic memory. Oxford University Press, Oxford, UK.
- Tulving, E., Markowitsch, H.J., Kapur, S., Habib, R., and Houle, S. 1994. Novelty encoding networks in the human brain: Positron emission tomography data. Neuroreport 5: 2525–2528. [DOI] [PubMed] [Google Scholar]
- Wagner, A.D. 1999. Working memory contributions to human learning and remembering. Neuron 22: 19–22. [DOI] [PubMed] [Google Scholar]
- Zeineh, M.M., Engel, S.A., and Bookheimer, S.Y. 2000. Application of cortical unfolding techniques to functional MRI of the human hippocampal region. NeuroImage 11: 668–683. [DOI] [PubMed] [Google Scholar]