Abstract
Although much has been learned about the role of the amygdala in Pavlovian fear conditioning, relatively little is known about an involvement of this structure in more complex aversive learning, such as acquisition of an active avoidance reaction. In the present study, rats with a pretraining injection of the N-methyl-D-aspartate (NMDA) receptor antagonist, 2-amino-5-phosphonopentanoic acid (APV), into the basolateral amygdala (BLA) were found to be impaired in two-way active avoidance learning. During multitrial training in a shuttle box, the APV-injected rats were not different from the controls in sensitivity to shock or in acquisition of freezing to contextual cues. However, APV injection led to impaired retention of contextual fear when tested 48 h later, along with an attenuation of c-Fos expression in the amygdala. These results are consistent with the role of NMDA receptors of the BLA in long-term memory of fear, previously documented in Pavlovian conditioning paradigms. The APV-induced impairment in the active avoidance learning coincided with deficits in directionality of the escape reaction and in attention to conditioned stimuli. These data indicate that normal functioning of NMDA receptors in the basolateral amygdala is required during acquisition of adaptive instrumental responses in a shuttle box but is not necessary for acquisition of short-term contextual fear in this situation.
Pavlovian fear conditioning has become a widely used model system to study neural substrates underlying aversive learning (LeDoux 1998; Fanselow and LeDoux 1999). Using this approach, a considerable body of data has been accumulated that indicates the essential role of the nuclei of the amygdala, especially the lateral and basolateral nuclei, in associative changes during fear conditioning (Quirk et al. 1995; Maren and Fanselow 1996; Maren et al. 1996a; Schafe et al. 2000). However, the data based on inhibitory avoidance learning support the argument that the amygdala mainly modulates the consolidation of fear memories in other brain structures (McGaugh et al. 1995; Roozendaal et al. 1997; Cahill and McGaugh 1998). One of the possible resolutions of this dispute is that the amygdala may play a dual role in these paradigms; namely, that in classical fear conditioning, the amygdala serves as the site of plasticity underlying fear learning, whereas in inhibitory avoidance learning, the amygdala modulates the strength of aversive memory located elsewhere (Wilensky et al. 2000).
Notably, both the fear conditioning and the inhibitory avoidance paradigms consist of a very low number of trials, thus limiting an opportunity to assess the role of the amygdala in a complex relation between fear processing and an acquisition of the instrumental reaction. One of the approaches to address this question involves more complex models of aversive learning, such as the active avoidance paradigm, which includes fear conditioning and demands dozens of trials to learn the instrumental response (for review, see Bolles 1979; Mineka 1979).
The process of learning the two-way active avoidance reaction can be dissected into well-defined and separated stages, starting from the initial execution of an inborn fleeing reaction from shock, then through the stage of acquisition of the instrumental escape response, and finally to the performance of an avoidance reaction (Savonenko and Zielinski 1998; Savonenko et al. 1999b,c). Detailed analysis of overt behaviors during learning revealed that acquisition of the active avoidance response was accompanied by attention and preparatory responses to the conditioned stimulus and was negatively correlated with freezing behavior (Savonenko et al. 1999a).
Lesions located into the various nuclei of the amygdala had been shown to impair the acquisition of the two-way active avoidance reaction (Werka 1997; Werka and Zielinski 1998). However, the study of plasticity-related neuronal activation in the amygdala during the same kind of behavioral training, as revealed by c-Fos immunocytochemistry, showed an apparent discrepancy with the results of lesions (Savonenko et al. 1999a). Namely, a pronounced activation of c-Fos expression in the basolateral amygdala (BLA), whose lesions had deteriorating effects on two-way avoidance learning, did not correlate with the acquisition of an instrumental reaction, but only with intertrial reactions that reflected the anticipatory anxiety of the animals. This could indicate that plastic long-term changes occurring in the BLA during instrumental aversive conditioning may reflect memory for fear-related information but not the memory of the instrumental reaction itself (for discussion, see Savonenko et al. 1999a). On the other hand, participation of the BLA in long-term retention of fear does not preclude the possible role of this structure in working memory circuits during acquisition of an adaptive instrumental reaction.
Blocking of N-methyl-D-aspartate (NMDA) receptors is another approach to assess the role of the amygdala by a temporary inactivation of these receptors during instrumental defensive learning. Investigations into the cellular mechanisms of conditioned stimulus (CS)–unconditioned stimulus (US) association revealed that NMDA receptors in the BLA are necessary for acquisition and expression of Pavlovian fear conditioning in rats (Maren et al. 1996b; Lee and Kim 1998; Walker and Davis 2000). It has been proposed that glutamate receptor-dependent long-term potentiation (LTP) may underlie neural plasticity of fear circuitry (McKernan and Shinnick-Gallagher 1997), and the effect of the NMDA receptor antagonist, 2-amino-5-phosphonopentanoic acid (APV), on fear conditioning appears to be mediated through a blockade of LTP induction (Maren and Fanselow 1995). However, several studies had also shown effects of APV on normal synaptic transmission (Chapman and Bellavance 1992; Li et al. 1998), indicating that APV effects on fear conditioning may be mediated by attenuation of normal synaptic transmission of CS- and/or US-related information (Lee et al. 2001; Rodrigues et al. 2001).
To get further insight into the involvement of the BLA amygdala during instrumental defensive conditioning, we have decided to use a pretraining intra-amygdala APV infusion as an approach that had been shown to impair acquisition of fear in classical Pavlovian paradigms. To dissociate the effects of APV infusion on fear conditioning and instrumental learning, a detailed behavioral analysis was applied with a continuous monitoring of freezing reaction during the CS and the intertrial interval. The instrumental learning was characterized by acquisition of a preparatory response as orientation of the body to the opening, by directionality of the escape reaction, and by acquisition of an avoidance reaction. The incidence of attention reaction to the CS was also recorded during every trial. We have also used c-Fos immunomapping of amygdalar nuclei to study neuronal activation related to the manipulations used.
RESULTS
Histological Verification of Infusion Sites
To verify the location of the injection cannulae and thus to ascertain the proper injection sites, we have examined brain sections from all the animals under study. This analysis revealed that all but one rat in the APV group received appropriate bilateral placement of cannulae into BLA (see Fig. 1 for representative placement). One rat from the vehicle group displayed an extensive damage in the area of the left external capsule. The behavioral data obtained for both of these animals were excluded from the statistical analyses. Comparison of APV- and vehicle-infused brains showed no difference in tissue structure around the infusion sites.
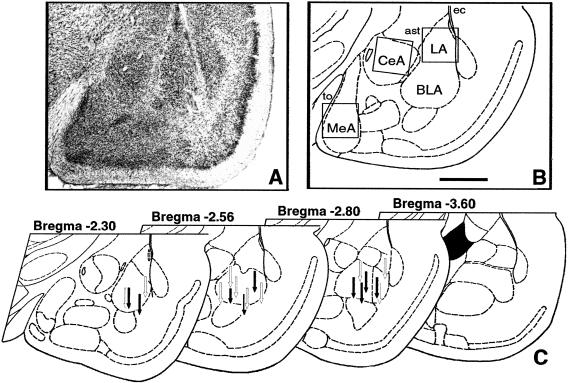
Figure 1.
Cannula placements. (A) Photomicrograph of Nissl-stained section of left amygdala in rat injected with APV. (B) Schematic representation of the same section. Inserted squares represent the areas shown on Figure 8 for examples of c-Fos immunostaining. Scale bar, 1 mm; (to) tractus opticus; (ec) external capsule; (ast) amigdalostriatal area. (C) Cannula tip placements from rats infused with vehicle (open arrows) or 5 μg of APV (filled arrows). Only rats with cannula tips at or within the boundaries of basolateral nuclei were included in the data analysis.
Acquisition of the Avoidance Reaction
Three groups of rats were used in the study to discriminate between effects of APV treatment and vehicle injection: control, vehicle, and APV groups. To assess the contribution of the NMDA receptors to the processing of stimuli of different modality, we used two conditioned stimuli, Noise and Darkness, presented in a semirandom order.
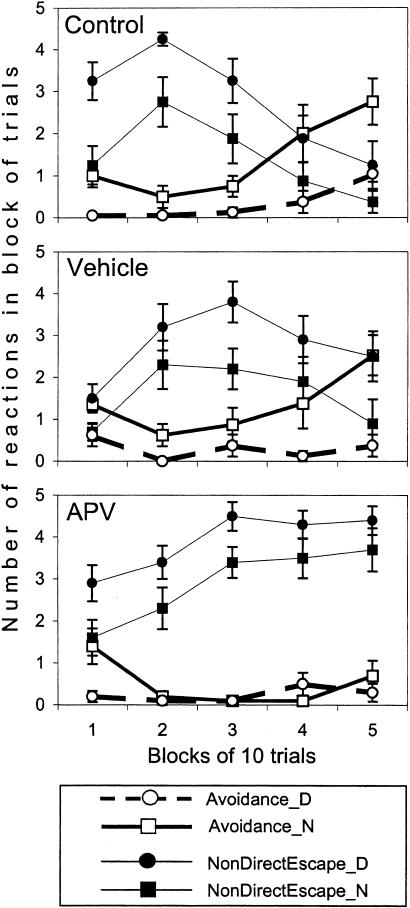
The acquisition of avoidance reactions was observed in the control and vehicle groups as a significant increase in the percentage of these reactions in the course of the session, whereas there was no such increase in the APV group (Fig. 2; effect of block, F(4, 100) = 6.51, p < 0.001; and group × block interaction, F(8, 100) = 2.09, p < 0.05). Consistent with the previous studies (Zielinski et al. 1993; Werka and Zielinski 1998), auditory CS (Noise) was more effective than visual CS (Darkness) in eliciting avoidance (effect of CS modality F(1,25) = 35.28, p < 0.0001). However, in contrast to the control and vehicle groups, the presentation of more “potent” auditory CSs did not facilitate learning in APV-injected rats (group × CS modality interaction F(2,25) = 4.22, p < 0.03).
Figure 2.
An acquisition of the two-way active avoidance reaction in control (top panel), vehicle (middle panel), and APV group (low panel). Thick lines represent the number of avoidance reactions to acoustic (Noise, N) or visual (Darkness, D) conditioned stimuli in consecutive blocks of trials. Thin lines represent the number of nondirectional responses that were observed as a chaotic jumping between the walls or to the ceiling of the shocked compartment before entering the opposite safe compartment. A decrease in the number of nondirectional responses to the end of the session (as it can be seen in controls but not in APV group) indicates an acquisition of escape reaction with an appropriate directionality and facilitates the learning of the avoidance reaction. Data are expressed as means and SEM.
Escape Behavior During Two-Way Active Avoidance Training
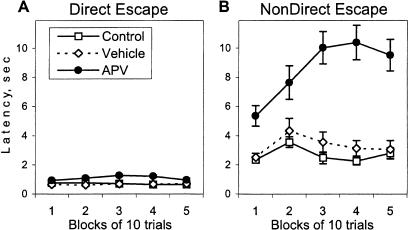
The deterioration in the acquisition of the avoidance reactions observed in the APV group might result from an impairment of one of the preceding stages of learning. The acquisition of an appropriate directionality of escape reaction is necessary for successful learning of the two-way avoidance reaction. As it can be seen in Figure 2 (top panel), the acquisition of the avoidance reaction in the control rats was detected in blocks of trials with a robust decrease in frequency of nondirectional escape responses. The nondirectional escape responses were observed as a jumping between walls or to the ceiling before crossing to the opposite, safe compartment. Another type of escape response, a directional escape, consisted in movement to the safe compartment with the shortest trajectory and resulted in very short escape latencies (Fig. 3A). The acquisition of the appropriate directionality in escaping shock facilitated learning of the two-way avoidance reaction. A similar pattern of changes was observed in the vehicle group (Fig. 2, middle panel). However, APV-injected rats showed significantly more nondirectional escape responses than the two other groups (effect of group, F(2,25) = 5.36, p < 0.01; Fig. 2, lower panel). Moreover, in the APV group, there was no inhibition of this reaction to the end of the session (effect of block, F(4, 100) = 9.56, p < 0.0001; and group × block interaction, F(8, 100) = 8.51, p < 0.0001). The latency of nondirectional responses in APV-injected rats was significantly longer than in the two other groups (Fig. 3B; Kruskal-Wallis ANOVA, H(2,636) = 182.53, p < 0.001) and increased in the course of the session (H(4,296) = 10.02, p < 0.05). However, APV rats escaped from shock as fast as the two other groups if the directionality of escape was appropriate (Fig. 3A), indicating no significant effects of APV injection on sensitivity to shock.
Figure 3.
Effect of APV on the latency of (A) directional and (B) nondirectional escape responses. In all groups of rats, the directional escape (defined as an escape from the shocked compartment with the shortest trajectory) was characterized by significantly shorter latencies than the nondirectional escape response (Kruskal-Wallis ANOVA, H(1, 1122) = 764.28, p < 0.0001). APV-injected rats were significantly different from the control groups only in the latency of nondirectional escape reactions (see text). Because there was no significant effect of modality of CS on escape latency, data are expressed as mean escape latencies for both conditioned stimuli.
In all groups, including APV-injected rats, the modality of the CS significantly affected escape behavior (F(1,25) = 59.26, p < 0.0001). The acoustic CS, Noise, was more potent in eliciting the escape response with an appropriate directionality than the visual CS, Darkness. The fact that APV-injected rats were sensitive to modality of the CS is consistent with a previous report that lesion of the BLA does not disrupt the effects of CS modality in the same learning paradigm (Werka and Zielinski 1998). Moreover, this fact indicates that inability of APV-injected rats to show modality-specific facilitation in acquisition of the avoidance reaction (see previous section of Results) was not caused by the deficit in sensitivity to CS modality.
Overt Behaviors During the CS and Intertrial Intervals
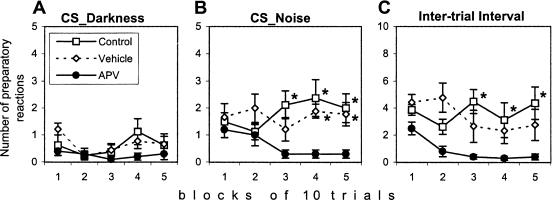
Preparatory reaction observed as orientation of the body toward the opposite compartment can facilitate avoidance or escape responses (Savonenko et al. 1999b). The APV group showed a significant deficit in preparatory responses during the acoustic CS (effects of group, F(2,26) = 7.64, p < 0.005; and group × modality interaction, F(2,26) = 3.50, p < 0.05). The absence of between-group differences during the visual CS was caused by a low level of preparatory responses in all groups (effect of modality, F(1,26) = 36.86, p < 0.0001; Fig. 4 A,B). The deficit of the APV group in this type of behavior was also revealed during intertrial intervals (effect of group, F(2,24) = 7.20, p < 0.005; Fig. 4C). These data are consistent with results on APV-induced deficiency in directionality of the escape because both, directional escape reaction and preparatory reaction, demand the correct discrimination of dangerous and safe compartments.
Figure 4.
Effect of APV injection on the preparatory reaction during (A) Darkness or (B) Noise conditioned stimuli or during (C) the intertrial interval. The reaction of orientation of the body or head to the opening was significantly suppressed in the rats of the APV group during presentation of the acoustic CS as well as during the intertrial interval. Values represent the mean ± SEM. (*) Significant differences from the APV group in separate blocks of trials as a result of Newman-Keuls post hoc test, p < 0.05.
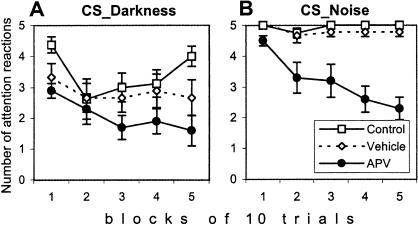
An even more dramatic effect of APV injection was observed when an attention reaction to the onset of CS was considered (Fig. 5). The attention reaction was defined as any change in ongoing behavior during the first second of CS presentation (initiation of the preparatory reaction, movement of a head, tail up reaction, or a break of any preceding movement). Because the attention reaction consisted of the elements of several reactions, it was not an independent behavioral variable. However, it was the most sensitive measure to reflect a general reactivity to the CS without taking into account what exact type of behavior will be elicited by the CS. The deficit of APV-injected rats in attention reactions was significant for both modalities (effect of group, F(2,24) = 15.39, p < 0.0001) and increased gradually during the training session (effect of block, F(4,96) = 6.72, p < 0.0001; and group × block interaction, F(8,96) = 2.30, p < 0.05).
Figure 5.
Attention reaction to (A) Darkness or (B) Noise conditioned stimuli was suppressed after APV injection in the basolateral nucleus of the amygdala. The effect of APV was observed for conditioned stimuli of both modalities and became more pronounced in the course of the session.
Freezing During the CS and Intertrial Intervals
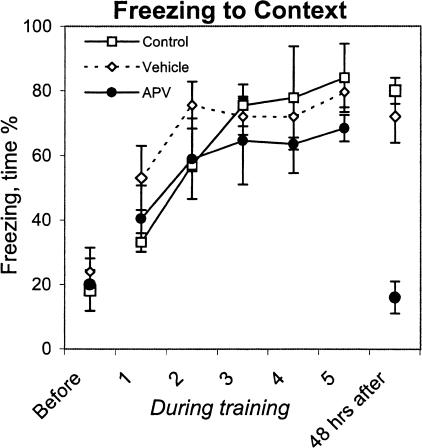
Freezing was analyzed separately for intertrial intervals and CS presentation. There were no between-group differences in freezing during the intertrial interval (F(2,24) = 0.70, p = 0.52; Fig. 6). APV-injected rats, similar to rats from the control and vehicle groups, increased time spent in freezing from the first to the second block of 10 trials, and then the level of freezing remained the same until the end of the session (effect of block, F(4,96) = 9.78, p < 0.001). These data indicate that APV injection into the BLA does not prevent acquisition of freezing to contextual cues when tested in the instrumental multitrial paradigm.
Figure 6.
Freezing to contextual cues measured before, during (1–5 blocks of trials), and 48 h after training. APV-injected rats showed a deficit in freezing only when tested 48 h after the training.
In contrast to the intertrial interval, freezing during the CS when measured in the instrumental paradigm can be disrupted by an avoidance reaction. Thus, only trials that were not terminated by an avoidance reaction were taken into analysis. Similar to the results from the intertrial interval, there was no between-group difference in freezing to CS (effect of group, F(2,24) = 0.33, p = 0.72). A significant effect of block of trials (F(4,96) = 6.67, p < 0.001) consisted of an increase of time spent in freezing from the first to the second block of trials without further changes until the end of the session.
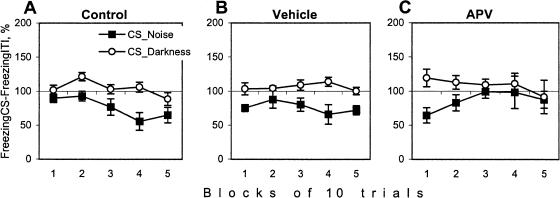
Because the CS was presented on the background of contextual cues, the increase in freezing to the CS can be a result of fear to contextual cues. Then, to estimate a relative impact of the CS on freezing behavior, we compared the percentage of time spent freezing during the intertrial interval and the CS (Fig. 7). In the control groups, the visual CS did not significantly increase freezing, probably because of a ceiling effect. The presentation of the acoustic CS decreased the level of freezing as compared with the intertrial interval (although not significantly, p < 0.08). This partial inhibition of freezing during the acoustic CS is consistent with the fact that in the control and vehicle groups, this stimulus was more potent in eliciting the preparatory response and facilitating the acquisition of the avoidance reaction. Importantly, in APV-injected rats, the inhibition of freezing was observed in the first block of trials but disappeared in the course of the session (Fig. 7C). Thus, to the end of the training, CS presentation in the APV group did not exert any additional effects on freezing behavior as compared with the intertrial interval.
Figure 7.
Freezing during Darkness or Noise conditioned stimuli in (A) control, (B) vehicle, and (C) APV groups. Freezing during the CS is expressed relative to the level of freezing during the intertrial interval (taken as 100% in every block of trials). Only trials that were not terminated by avoidance reaction were analyzed (see text).
Overt Behaviors After 48-h Delay
After 48 h of delay, the rats were placed in the same shuttle box in which they were trained, and overt behaviors were observed during a 26-sec interval (an equivalent of the longest intertrial interval during the active avoidance training). All control animals oriented their heads and bodies to the opening between compartments of the apparatus and then froze for the rest of the observation period. Freezing to contextual stimuli in rats from the vehicle group was not different from the controls. In contrast, time spent in freezing by APV-injected rats was significantly less than in two previous groups (F(2,11) = 17.78, p < 0.001) and, moreover, was not different from that observed before training (see Fig. 6, before and after training).
Effect of APV on the Training-Induced c-Fos Activation in the Amygdala
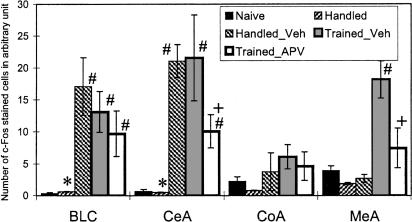
To investigate amygdala regions that are activated after a single training session of the two-way active avoidance, we analyzed c-Fos immunolabeling of this brain region. In the naive, untrained animals, there was a very low level of c-Fos expression (Fig. 8). To assess the effects of handling and injection on c-Fos activation, the animals from the handled group were injected with vehicle only in the left amygdala. 3-way ANOVA of c-Fos expression in naive and handled rats revealed a significant effect of the side of injection (F(1,4) = 9.98, p < 0.05) and group × side interaction (F(1,4) = 10.12, p < 0.05). The Newman-Keuls post hoc test showed significant differences between the left and right amygdala for basolateral (BLC) and central (CeA) nuclei of handled rats (Fig. 9). This indicated that handling alone did not alter c-Fos levels but vehicle injection in the handled animals produced an increase of c-Fos expression in the nuclei adjacent to the trace of cannulae.
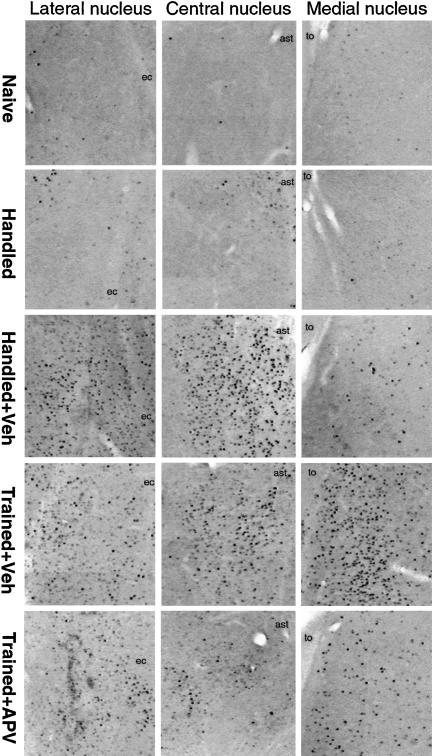
Figure 8.
Representative examples of c-Fos immunostaining in the lateral, central, and medial nuclei of amygdala in control rats (naive, handled, and handled and injected with vehicle) and in trained rats injected with vehicle or APV. For schematic location of areas shown, see Figure 1B. (to) Tractus opticus; (ast) amygdalostriatal area; (ec) external capsule.
Figure 9.
c-Fos expression in basolateral complex (BLC), central (CeA), cortical (CoA), and medial (MeA) amygdalar nuclei in control and experimental groups of rats. The data represent the average number of c-Fos immunostained cells of left and right nuclei except the handled group, where the data from right (Handled) and left (Handled_Vehicle) nuclei were presented separately. (*) Significant differences between right and left (vehicle-injected) sides in group of handled rats; (#) significant differences from naive rats; (+) significant differences from Trained-Vehicle group, p < 0.05 in each case (Newman-Keuls post hoc tests; see text for results of ANOVAs).
To assess the effect of training and APV injection on c-Fos activation in amygdala, the data from naive rats and handled rats injected with vehicle (only from the left injected side) as well as from trained animals injected with vehicle or APV were included in 4 (groups) × 4 (nuclei) ANOVA. The analysis revealed the significant effect of group (F(3,11) = 3.76; p < 0.05), nuclei (F(3,33) = 4.95; p < 0.01), and interaction (F(9,33) = 2.47; p < 0.05). In the injected animals, irrespective of whether they were trained or not, there was a marked c-Fos expression observed close to the injection site, that is, BLC and CeA nuclei (see Fig. 9 for the results of post hoc tests). In the APV-injected animals, c-Fos expression in these nuclei was less robust (significantly in CeA). On the other hand, in both groups of the trained rats (APV- and vehicle-injected), an increase in c-Fos expression was observed in the amygdala regions—such as the medial area—that were distant to the injection site. The training-induced increase in c-Fos expression in the medial amygdala was markedly diminished in the APV-treated animals, resulting in significant differences between the APV and vehicle groups (Figs. 8 and 9).
DISCUSSION
The results of the present study indicate that pretraining infusion of the NMDA receptor antagonist APV into the BLA impairs the acquisition of the two-way active avoidance reaction. APV-injected rats failed to acquire the appropriate directionality of the escape reaction and showed additional deficits in attention to conditioned stimuli. Importantly, APV did not prevent the acquisition of freezing to contextual cues in the course of the avoidance training; however, it dramatically deteriorated the retention of contextual fear when tested 48 h after the training. This deficit in the long-term retention of conditioned fear coincided with a significant attenuation of c-Fos activation in the amygdala, indicating that NMDA receptor activation and Fos induction could be important for the retention of contextual fear in the two-way active avoidance procedure.
As an instrumental defensive paradigm, the active avoidance procedure includes the strong component of classical fear conditioning (for review, see Bolles 1979; Mineka 1979). Because APV injection into the BLA has been shown to ameliorate the acquisition of conditioned fear tested in Pavlovian conditioning (Maren et al. 1996b; Lee and Kim 1998), APV-induced impairment in the two-way active avoidance learning may be due to a detrimental effect on the fear processing during training. However, our APV-injected rats did acquire fear to contextual cues measured by freezing reaction and were not different in this behavior from rats of control and vehicle groups. Considering that the active avoidance training was a multitrial procedure, this fact is consistent with observations on dynamics of postshock freezing in a multitrial Pavlovian conditioning paradigm. Namely, rats injected with APV into the BLA increased their level of postshock freezing across three (Maren et al. 1996b; Lee and Kim 1998) and 10 (Lee et al. 2001) training trials. Moreover, rats with pretraining neurotoxic BLA lesions did show the immediate postshock freezing similar to sham operated controls if the training consisted of 50 trials (Maren 2001). All these data support the idea that an alternative neural system can be recruited to mediate fear conditioning if the function of the BLA is impaired (Cahill et al. 2000; Maren 2001).
Whatever extra- or intra-amygdalar neural system is responsible for the acquisition of immediate fear reactions when NMDA receptors in BLA are blocked, it cannot support a long-term fear retention. In this study, APV infusion dramatically attenuated the retention of contextual fear when tested after a 48-h delay. This indicates that NMDA receptors in the BLA are required for long-term memory of contextual fear in the complex defensive instrumental task, as it was shown in the Pavlovian conditioning paradigm (Kim et al. 1992; Fanselow and Kim 1994; Maren et al. 1996b; Lee and Kim 1998; Walker and Davis 2000).
The results on c-Fos activation in amygdalar neurons in response to NMDA receptor blockade indicate that the effect of APV on long-term retention of fear can be mediated by inhibition of an immediate early genes expression, namely, c-Fos. This view is consistent with several studies showing an association between NMDA activity and Fos regulation in different models of synaptic plasticity (Cole et al. 1989; Herrera and Robertson 1990; Aronin et al. 1991) as well as in long-term memory formation (for reviews, see Kaczmarek and Chaudhuri 1997; Kaczmarek 2002). Furthermore, previous studies indicated that during two-way active avoidance training, c-Fos activation is associated with the acquisition of new memories (Nikolaev et al. 1992; Savonenko et al. 1999a; Radwanska et al. 2002). That is, expression of c-Fos in the amygdala has been limited to a stage of initial acquisition but not asymptotic performance of the two-way active avoidance reaction (Nikolaev et al. 1992). Activation of c-Fos during this acquisition period has coincided with induction of phosphorylated extracellular signal-regulated kinase (P-ERK) (Radwanska et al. 2002), which has been linked to mechanisms of memory consolidation during Pavlovian fear conditioning (Schafe at al. 2000). Finally, c-Fos expression in the amygdala has correlated with measures of emotional learning, but not sensory stimulation (duration of footshock) or instrumental reactions (escape or avoidance; Savonenko et al. 1999a).
In contrast to the previous studies (Nikolaev et al. 1992; Savonenko et al. 1999a; Radwanska et al. 2002), a pattern of c-Fos expression in the recent experiment has been additionally affected by two factors: by a blockade of NMDA receptors and by an infusion of a solution into the brain. An interference of these two factors was most obvious in the nucleus closely adjacent to the cannulae trace, BLA. Similar observations on an injection-related c-Fos activation have been previously reported in rats and chicks (Kaczmarek et al. 1988; Freeman and Rose 1995). In our experiment, maximal concentrations of APV, which has been achieved during infusion into the BLA, were insufficient to reduce the injection-related c-Fos activation in this nucleus, implicating a possible involvement of NMDA-independent mechanisms. In the CeA, a nucleus that was more distant from the injection site than BLA, an injection-related c-Fos activation has still been observed; however, it was significantly reduced by APV. Considering that the CeA has rich afferent connections with the BLA (Pitkanen et al. 1997), the effect of APV in lowering c-Fos expression in the CeA is likely to be mediated by changes in BLA output neuron activity. Alternatively, one may suggest a direct effect of APV in lowering c-Fos expression in the CeA caused by a possible APV diffusion from the injection site. It is important to note that c-Fos activation in the CeA was entirely a result of infusion in the BLA because training in the two-way active avoidance paradigm does not activate c-Fos expression in the CeA when tested without infusion (Savonenko et al. 1999a). However, the injection-induced c-Fos up-regulation in the BLA and CeA affected neither the avoidance learning nor the retention of contextual fear in vehicle-injected rats.
The effects of training and APV on c-Fos expression were clearly revealed in the medial amygdala. In this nucleus, which was most distant to the cannulae trace, there was no effect of injection on c-Fos expression. The training-induced c-Fos activation in the MeA was previously reported after one-trial contextual fear conditioning (Milanovic et al. 1998; Radulovic et al. 1998) as well as after one session of two-way active avoidance learning (Savonenko et al. 1999a). Moreover, in the last paradigm, a training-induced c-Fos activation in the MeA significantly correlated with c-Fos expression in the BLA (Savonenko et al. 1999a), indicating that these nuclei may function in concert during the two-way active avoidance learning. In the present experiment, APV infusion has ameliorated the training-induced c-Fos activation in the MeA. Considering that the infusion site was remote from the medial amygdala, the c-Fos lowering effect of APV in this nucleus could be mediated by changes in intra-amygdala circuits. The lateral nucleus is known to have the most extensive intra-amygdaloid projections, including the MeA (Pitkanen and Kemppainen 2002), which provide a good anatomical background for the observed effects. It is noteworthy that, because there was no injection-related c-Fos activation in the MeA, vehicle infusion in the BLA appeared not to alter the intra-amygdala circuits, which could be important for a preserved avoidance and fear conditioning in the vehicle-injected rats.
Thus, in the two-way active avoidance paradigm, a blockade of NMDA receptors in the BLA altered the pattern of c-Fos expression in the amygdala and resulted in a deficit of a long-term retention of contextual fear, whereas the acquisition of short-term contextual fear has been preserved. In multitrial Pavlovian fear conditioning, short-term contextual fear has also been preserved after the pretraining BLA lesion, but a deficit in cue-related fear conditioning has not been overcome even after extensive overtraining (Maren 1999). This indicates that during a multitrial training, deficits in BLA function may have different outcomes on contextual and cued fear conditioning.
It can be suggested that the blockade of NMDA receptors in BLA during the two-way active avoidance training disrupted a CS–US association as was shown in the Pavlovian conditioning paradigm. A poor CS–US association would result in an inability of APV-injected rats to differentiate between a safe and unsafe compartment, and delay a rate-limiting step of learning, the acquisition of an appropriate directionality of escape reaction (Bignami et al. 1985). The results presented herein support this view. CS presentation in the APV group did not exert any additional effects on freezing behavior as compared with the intertrial interval. Moreover, APV-injected rats were severely impaired in the acquisition of directional escape reaction and showed significantly more nondirectional escapes than other groups. Importantly, the delay in acquisition of appropriate directionality of the escape reaction increased the amount of unconditioned stimulation received by APV-injected rats. This may further augment contextual fear and result in behavior similar to inhibitory avoidance or place-avoidance responses.
A dramatic deficit in the attention reaction to the CS observed in APV-injected rats could be one of the mechanisms of a poor CS–US association. Attention to auditory and visual CS has been shown to be regulated by the amygdala, and especially by the central nucleus, in the appetitive conditioning paradigm (Holland and Gallagher 1993,1999). However, the question regarding the differential impact of spatially adjacent amygdalar nuclei on attentional processes cannot be ascertained in the present study because of possible APV diffusion, and deserves further investigation.
Thus, in the setting of multitrial training, a differential effect of NMDA receptor blockade in BLA on cued and contextual fear conditioning may be a primary mechanism of impaired two-way active avoidance learning. Studies on classical fear conditioning have shown that pretraining effects of APV in the amygdala could be attributable to a disruption of routine synaptic transmission instead of, or in addition to, a disruption of plasticity (Maren et al. 1996a; Lee and Kim 1998; Lee at al. 2001; see also Rodrigues et al. 2001 for discussion). Impaired acquisition of cued fear conditioning in our APV-treated rats indicated that normal synaptic transmission in the BLA may also have been disrupted as a result of APV infusion. In this case, the effects of APV on plasticity-related c-Fos expression in the amygdala can be interpreted as secondary to the effects on normal synaptic transmission. Based on the present results, one may hypothesize that the normal functioning of NMDA receptors in the BLA during the two-way active avoidance training is required for an appropriate representation of CS–US information in a circuitry responsible for acquisition of the instrumental responses. This view does not necessarily require the participation of NMDA receptors of the BLA in mechanisms of storage of “instrumental” memories, and is consistent with our previous data on the absence of correlation between c-Fos activation in the amygdala and instrumental behavior (Savonenko et al. 1999a). Importantly, however, the present results on the APV-induced specific deficit in long-term retention but not acquisition of contextual fear support the hypothesized role of the amygdala in long-term storage of “fear” memories (LeDoux 1998; Fanselow and LeDoux 1999).
MATERIALS AND METHODS
Subjects
The experiment was conducted on 37 adult male Möll-Wistar rats bred in the Nencki Institute, experimentally naive, and weighing 320–360 g. Subjects were kept in groups of four to five in home cages (43 cm long, 25 cm wide, 18.5 cm high), containing food and water available ad libitum. A natural light–dark cycle from external illumination was maintained. All behavioral testing was performed during the light phase of the cycle. The experiment was performed in strict accordance with recommendations of the European Union (86/609), disposition of the President of the Polish Republic, and approved by the Ethical Committee on Animals Research of the Nencki Institute. All efforts were made to minimize animal suffering and reduce the number of animals used.
Surgery and Drug Administration
Animals were anesthetized with chlorohydrate (360 mg/kg) and placed in a Kopf stereotaxic apparatus. The guide cannulae were made from 23-gauge stainless steel tubing. They were 14.0 mm long and beveled at the distal end to minimize tissue damage during implantation. Cannulae were held in place by a carrier attached to the stereotaxic arm and then cemented with dental acrylic. Skull screws provided an extra binding surface for the acrylic. Following implantation, cannulae were closed with removable wire stylets. The stereotaxic coordinates for implantation of the guide cannulae were as follows: Bregma, –1.0; lateral, ±4.8; from dura, –7.2, with the incisor bar set at +5.0 mm from the horizontal plane passing through the interaural line. Injection cannulae were cut from 30-gauge steel tubing and designed to protrude 2.0 mm beyond the tip of the guide cannulae to reach the basolateral amygdala (BLA). 2-Amino-5-phosphonopentanoic acid (DL-APV; Sigma Chemical Co.) was dissolved into an artificial CSF. This buffer solution was also used for vehicle infusion. Injection cannulae were connected by polyurethane tubing to a 10-μL Hamilton microsyringe mounted on a microinfusion hand-driven pump. The drug and vehicle were infused in a volume of 0.7 μL over 5 min and allowed to diffuse for a further minute. The infusions on the left and the right amygdala were administered consecutively.
Group Treatment
Before initiation of experimental procedures, 30 rats were randomly assigned to three groups: a control group (n = 10) and two groups for infusions (10 animals each). All rats were handled daily (5–6 min per rat) for 7 d before behavioral testing. The experimental groups received an intra-amygdala infusion of APV at the dose of 5 μg (group APV, n = 10) or vehicle (group Veh, n = 10). The rat was gently held, and the injection needle was inserted into the cannula with the stylet removed. Behavior training began 5 min after the stylets were replaced. After the training session, one-half of the rats from the APV and Veh groups were randomly assigned for the c-Fos immunomapping and the rest of the rats were used for behavioral testing in the same shuttle-box apparatus after 48 h of delay.
An additional two groups of rats were used to control for a basal level of c-Fos immunoreactivity in naive animals (n = 4) and for effects of handling and vehicle injection without behavioral training (n = 3). The last group was handled and underwent surgery in the same way as the APV and Veh groups, but received intra-amygdala infusion of vehicle only in one hemisphere.
Behavioral Apparatus
The shuttle-box apparatus was 62 cm long, 18 cm wide, and 29 cm high, with walls of opaque, white acrylic. The box was divided in half by a wall with a rectangular (7 cm wide, 10 cm high) opening situated on the grid-floor level permitting passage from one side of the shuttle box to the other. Each compartment was covered with a movable transparent acrylic ceiling and illuminated by a 5-W lamp mounted centrally just below the ceiling. The response of crossing through the opening was recorded by photocells mounted 4 cm to either side of the central partition, 5 cm above the floor level. The floor in each compartment was made of 16 stainless steel bars, 0.4 cm in diameter, located parallel to the central partition, 1.5 cm apart from each other. The shuttle-box apparatus was placed in a soundproof, dim lighted room. A low-light television camera (Panasonic) was mounted 50 cm above the shuttle box so it could include both compartments in its field of view. The sensitivity of the camera was high enough to create a clear image of the rat sitting in an unilluminated compartment when the main source of light was the lamp in the opposite compartment. The subjects' behavior was watched on a TV monitor in an adjoining room, where equipment for automatic programming of the experiment and recording of data was located.
Two-Way Active Avoidance Training
Each rat was first preliminarily habituated to the situational cues of the apparatus for 10 min on two consecutive days. A single training session started the day after the second habituation session and consisted of 50 trials. At the beginning of the session, the rat was placed in the left compartment of the shuttle box, close to and facing the end wall. After 20 sec, a trial started with conditioned stimulus (CS) onset and, 5 sec later, the scrambled shock of 1.6 mA nominal intensity activated the grid floor (unconditioned stimulus, US). Running to the opposite compartment within the 5-sec CS–US interval precluded the footshock, immediately terminated the CS, and was scored as an avoidance response. A similar response, but after the US onset, immediately coterminated the CS and US and was scored as an escape response. The intertrial intervals lasted 14, 20, or 26 sec (mean = 20 sec) and varied in a semirandom order. During intertrial intervals, subjects were permitted to move in any direction, so they could cross away from or back into the compartment in which they had been previously. The next trial always started in the compartment where the subject was located at the end of the ITI. The maximal shock duration was 30 sec.
Two conditioned stimuli of different modality were used in a semirandom order. The visual conditioned stimulus, Darkness (D), was provided by termination of the ceiling light in the compartment occupied by the rat while the illumination in the opposite compartment remained on. Just after a rat left the shock compartment, it was again lighted; both compartments were illuminated during intertrial intervals. The acoustic conditioned stimulus, Noise (70 dB), was provided by a white noise generator and delivered in the compartment occupied by the rat.
To analyze the long-term fear conditioning after the two-way active avoidance training, one-half of the animals from each group were placed in the same shuttle-box apparatus after 48 h of delay. Freezing and other overt behaviors were assessed for 26 sec. The duration of the period of observation was chosen based on the duration of the intertrial interval during the avoidance training. Freezing during CS presentation was not analyzed because after the active avoidance training, the CS acquires a property of eliciting the instrumental reaction.
Coding of Freezing Reactions
Freezing behavior, defined as the lack of any movement except that related to respiration, was scored continuously with a stop-watch by reviewing videotapes. Two observers who were uninformed about subject's treatment rated the behavior of every rat twice: first, only during CS presentation, and second, only during intertrial intervals. The periods when freezing was not scored were used to record data. Agreement between two observers (calculated as the Pearson correlation of duration of freezing) was highly significant (r = 0.93–0.97, ps < 0.001). The percentage of time spent in freezing was calculated for the intertrial interval and CS separately. Only trials that were not terminated by avoidance response were used to analyze the freezing during CS presentation.
Coding of Other Behavioral Reactions
All behavioral reactions have been described in detail previously (Savonenko et al. 1999a,b,c). The behavior of each rat was observed, coded sequentially, and noted by an experimenter blinded to the type of treatment. After 3 wk, video records from eight rats were selected randomly for code checking by the same observer. Code agreement (calculated by dividing [total agreements] by [total agreements added to total disagreements]) averaged 0.94 ± 0.01 (mean ± SEM). Reliability for each of the individual categories of behavior is presented below in square brackets.
The following behaviors were coded during CS presentation:
Avoidance response [checked automatically]—crossing the opening to the opposite compartment within the 5 sec of the CS–US interval.
Preparatory response during CS presentation [0.97]—turning of the body and orienting of the head toward the opening during CS presentation. The orientation of the body and head has to be maintained for at least 1 sec for the preparatory response to be coded except for the cases when preparatory response was followed by the avoidance response. If after the preparatory response has been coded, a movement of the body occurred that demolishes the orientation toward the opening, the dissipation of the preparatory response was recorded.
Attention reaction to the CS [0.95]—any change in ongoing behavior, which was observed during the first second of CS presentation, as initiation of the preparatory reaction, dissipation of freezing, or break of any previous activity. Because the reaction to CS onset included the elements of other behaviors, it was not considered as an independent category. However, this reaction was scored separately to ensure the possibility to analyze whether the CS onset generated any change in overt behavior.
The overt behavior during US presentation was discriminated into two types of escape responses:
A directional escape response [0.92]—an escape from the shocked compartment by one of the shortest trajectories. If before a shock presentation the body of the rat was oriented toward the opening, the directional escape response was recorded if the rat ran straight to the opening. If there was no preparatory response before a shock presentation, the directional escape response was coded if the trajectory included the turning of the body toward the opening followed by running into the opening.
A nondirectional escape response [0.91]—an escape response, observed as a jumping between walls or to the ceiling of the occupied compartment before running to the opposite safe compartment. The cases when the rat failed to escape to the opposite compartment were very rare (0.2% in the control group and 2.3% in the APV group). These reactions were included in the category of the nondirectional escape responses and were not analyzed separately.
Considering that the escape latency in the shuttle box is affected by the modality of the CS (Zielinski et al. 1993; Werka and Zielinski 1998), no ad hoc arbitrary criterion of latency was used to discriminate the directional and nondirectional escape responses during coding. The latencies of two types of escape responses were analyzed after coding (see Results).
During the intertrial interval, the incidences of preparatory responses [0.95] were recorded. Operational definition of this response was the same as for that observed during CS presentation (see above). The other behaviors that were scored but are not reported here included grooming, sniffing, and biting the steel bars.
Behavioral Measures
Freezing behavior was expressed as a percentage of time during the CS or intertrial interval and averaged for every block of 10 trials. The avoidance and escape responses were characterized by a frequency of the appropriate responses in consecutive blocks of 10 trials. Because avoidance response and directional and nondirectional escape responses give together 100% of crossings, only data of the avoidance and nondirectional escape responses were presented. The escape responses were also characterized by latencies averaged separately for the CS of different modality. The preparatory behavior during CS was characterized by the number of preparatory responses counted separately for each CS in consecutive blocks of 10 trials. Because the incidences of dissipation of the preparatory response were practically not observed during CS presentation (3 cases per 1396 observations), the index of the preparatory response during the CS was expressed as a percentage of trials with this behavior. During the intertrial interval, there was a possibility to observe more than one preparatory response. Only preparatory responses that did not disappear at the end of the intertrial interval and, consequently, could directly affect the behavior during CS and US presentation, were analyzed. This overt behavior was also expressed as a percentage of trials in consecutive blocks.
Histology
After the end of the experiment, all rats were anaesthetized with chloride hydrate overdose and perfused intracardially with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed and stored in the same fixative at 4°C for 24 h and then in 30% sucrose with 0.02% sodium azide at 4°C until needed. The appropriate parts of the brain were slowly and gradually frozen in a heptane/dry ice bath and sectioned at 45 μm on a cryostat. Histological verification of cannulae location was performed by using Nissl-stained sections (Bures et al. 1983).
c-Fos Immunocytochemistry
The expression of c-Fos was assessed essentially as described before (Kaminska et al. 1996). The rats that were used for immunocytochemistry were anaesthetized and sacrificed 2 h after the beginning of the training session. The brain sections were washed three times in PBS (pH 7.4), incubated 10 min in 0.3% H2O2 in PBS, washed twice in PBS, then incubated with a polyclonal antibody (anti-c-Fos, 1:1000, Santa Cruz #sc-52) at 4°C for 48 h in PBS with azide (0.01%) and normal goat serum (3%). After that, the sections were washed three times in PBS with Triton X-100 (0.3%; Sigma), incubated with goat anti-rabbit biotinylated secondary antibody (1:1000; Vector) in PBS/Triton and normal goat serum (3%) for 2 h, washed three times in PBS/Triton, incubated with avidin–biotin complex (1:1000, 1:1000; Vector, in PBS/Triton) for 1 h, and washed three times in PBS. The immunostaining reaction was developed using the glucose oxidase–DAB–nickel method. The sections were incubated in PBS with DAB (0.05%), glucose (0.2%), ammonium chloride (0.04%), and ammonium nickel sulfate (0.1%; all from Sigma) for 5 min, then 10% (v/v) glucose oxidase (10 U/mL in H2O; Sigma) was added. The staining reaction was stopped by two to three washes with PBS. The sections were mounted on gelatin-covered slides, air-dried, dehydrated in ethanol solutions and xylene, and embedded in entellan (Merck).
The immunoreactivity in medial (MeA), central (CeA), and cortical (CoA) nuclei and basolateral complex (BLC, lateral and basolateral nuclei together) of amygdala were investigated. The image analysis was done with the aid of the image analysis system (MCID, IMAGING Research Inc.). c-Fos immunoreactivity was automatically scored as a number of c-Fos immunopositive cell nuclei per arbitrary unit. This measure was obtained by dividing the number of c-Fos grains in a particular region of amygdala by the area occupied by this region. Three parallel brain sections were analyzed. Because there was no significant effect of brain sections on c-Fos counts (repeated measures ANOVA), average c-Fos densities were used in further statistical analyses.
Four groups of animals were used for the assessment of the c-Fos activation in the amygdala: naive control (without any treatment, n = 4), handling and vehicle injection control (only left amygdala was injected, n = 3), vehicle-injected trained rats (n = 4) and APV-injected trained rats (n = 5).
Data Analysis
All data are represented as means and standard error of the means (SEM). The minimal level of significance was p < 0.05. The data of the behavioral testing were submitted to 2- or 3-way analysis of variance (ANOVA) with a between-subject factor of group (3 levels: Control, Veh, APV) and repeated measures factors of CS modality (2 levels: Darkness, Noise) and block of 10 trials (5 levels). The data of overt behaviors expressed as a percentage of reactions in block of trials were submitted to a square root transformation before applying ANOVA. Group differences for the latency of escape reactions were analyzed using nonparametrical statistical tests because the group data violated the assumption of normal distribution (i.e., failed the Kolmogorov-Smirnov test for normality, ps < 0.01). A Kruskall-Wallis nonparametric ANOVA was followed by individual Mann-Whitney tests with significance defined as p < 0.01 to compensate for multiple comparisons.
To assess the effect of handling and vehicle injection on c-Fos expression, the data of immunoreactivity were used in 3-way ANOVA (group: naive, handled) × side of the brain (injected, intact) × nuclei of amygdala (4 levels). To assess the effect of APV or Veh injection in the trained rats, the data of c-Fos expression were used in 2-way ANOVA (group: naive, handled-Veh, trained-Veh or trained-APV) × nuclei of amygdala (4 levels). The post hoc Newman-Keuls multiple comparison test followed the ANOVAs and applied to the significant main effects or its interactions. All statistical calculations were made using the package STATISTICA 5.0 for Windows.
Acknowledgments
The authors thank Alicja Markowska for helpful comments, Marek Rydz for technical assistance, Johanna Morton and Allegra Heinrichs for editorial help, and Seung-Hyun Woo and Kelly Straub for assistance with behavioral coding. This study was supported by the State Committee for Scientific Research (KBN, Poland, grant No. 6P04A03619). A.S. was supported by a short-term fellowship from the Committee for Aid for Neurochemistry, ISN and by the Nencki Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.58803.
References
- Aronin, N., Chase, K., Sagar, S.M., Sharp, F.R., and DiFiglia, M. 1991. N-Methyl-D-aspartate receptor activation in the neostriatum increases c-fos and fos-related antigens selectively in medium-sized neurons. Neuroscience 44: 409–420. [DOI] [PubMed] [Google Scholar]
- Bignami, G., Alleva, A., Amorico, L., De Acetis, L., and Giardini, V. 1985. Bidirectional avoidance by mice as a function of CS, US, and apparatus variables. Animal Learn. Behav. 13: 439–450. [Google Scholar]
- Bolles, R.C. 1979. Learning theory, 2nd ed. Holt, Rinehart and Winston, New York.
- Bures, J., Buresova, O., and Huston, J.P. 1983. Techniques and basic experiments for the study of brain and behavior, 2nd ed. Elsevier, Amsterdam–NY.
- Cahill, L. and McGaugh, J.L. 1998. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 21: 294–299. [DOI] [PubMed] [Google Scholar]
- Cahill, L., Vazdarjanova, A., and Setlow, B. 2000. The basolateral amygdala complex is involved with, but is not necessary for, rapid acquisition of Pavlovian `fear conditioning.' Eur. J. Neurosci. 12: 3044–3050. [DOI] [PubMed] [Google Scholar]
- Chapman, P.F. and Bellavance, L.L. 1992. Induction of long-term potentiation in the basolateral amygdala does not depend on NMDA receptor activation. Synapse 11: 310–318. [DOI] [PubMed] [Google Scholar]
- Cole, A.J., Saffen, D.W., Baraban, J.M., and Worley, P.F. 1989. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature 340: 474–476. [DOI] [PubMed] [Google Scholar]
- Fanselow, M.S. and Kim, J.J. 1994. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav. Neurosci. 108: 210–212. [DOI] [PubMed] [Google Scholar]
- Fanselow, M.S. and LeDoux, J.E. 1999. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23: 229–232. [DOI] [PubMed] [Google Scholar]
- Freeman, F.M. and Rose, S.P. 1995. MK-801 blockade of Fos and Jun expression following passive avoidance training in the chick. Eur. J. Neurosci. 7: 563–569. [DOI] [PubMed] [Google Scholar]
- Herrera, D.G. and Robertson, H.A. 1990. N-Methyl-D-aspartate receptors mediate activation of the c-fos proto-oncogene in a model of brain injury. Neuroscience 35: 273–281. [DOI] [PubMed] [Google Scholar]
- Holland, P.C. and Gallagher, M. 1993. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behav. Neurosci. 107: 246–253. [DOI] [PubMed] [Google Scholar]
- Holland, P.C. and Gallagher, M. 1999. Amygdala circuitry in attentional and representational processes. Trends Cogn. Sci. 3: 65–73. [DOI] [PubMed] [Google Scholar]
- Kaczmarek, L. 2002. c-Fos in learning: Beyond the mapping of neuronal activity. In Handbook of chemical neuroanatomy, vol. 19: Immediate early genes and inducible transcription factors in mapping of the central nervous system function and dysfunction (eds. L. Kaczmarek and H.A. Robertson), pp. 189–216. Elsevier, Amsterdam.
- Kaczmarek, L. and Chaudhuri, A. 1997. Sensory regulation of immediate-early gene expression in mammalian visual cortex: Implications for functional mapping and neural plasticity. Brain Res. Rev. 23: 237–256. [DOI] [PubMed] [Google Scholar]
- Kaczmarek, L., Siedlecki, J.A., and Danysz, W. 1988. Proto-oncogene c-fos induction in rat hippocampus. Brain Res. 427: 183–186. [DOI] [PubMed] [Google Scholar]
- Kaminska, B., Kaczmarek, L., and Chaudhuri, A. 1996. Visual stimulation regulates the expression of transcription factors and modulates the composition of AP-1 in visual cortex. J. Neurosci. 16: 3968–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.J., Fanselow, M.S., DeCola, J.P., and Landeira-Fernandez, J. 1992. Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behav. Neurosci. 106: 591–596. [DOI] [PubMed] [Google Scholar]
- LeDoux, J. 1998. Fear and the brain: Where have we been, and where are we going? Biol. Psych. 44: 1229–1238. [DOI] [PubMed] [Google Scholar]
- Lee, H. and Kim, J.J. 1998. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J. Neurosci. 18: 8444–8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H., Choi, J., Brown, T.H., and Kim, J.J. 2001. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J. Neurosci. 21: 4116–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Weiss, S.R., Chuang, D.M., Post, R.M., and Rogawski, M.A. 1998. Bidirectional synaptic plasticity in the rat basolateral amygdala: Characterization of an activity-dependent switch sensitive to the presynaptic metabotropic glutamate receptor antagonist 2S-α-ethylglutamic acid. J. Neurosci. 18: 1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren, S. 1999. Neurotoxic basolateral amygdala lesions impair learning and memory but not performance of conditional fear in rats. J. Neurosci. 19: 8696–8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren, S. 2001. Is there savings for Pavlovian fear conditioning after neurotoxic basolateral amygdala lesions in rats? Neurobiol. Learn. Mem. 76: 268–283. [DOI] [PubMed] [Google Scholar]
- Maren, S. and Fanselow, M.S. 1995. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J. Neurosci. 15: 7548–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren, S. and Fanselow, M.S. 1996. The amygdala and fear conditioning: Has the nut been cracked? Neuron 16: 237–240. [DOI] [PubMed] [Google Scholar]
- Maren, S., Aharonov, G., and Fanselow, M.S. 1996a. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: Absence of a temporal gradient. Behav. Neurosci. 110: 718–726. [DOI] [PubMed] [Google Scholar]
- Maren, S., Aharonov, G., Stote, D.L., and Fanselow, M.S. 1996b. N-Methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav. Neurosci. 110: 1365–1374. [DOI] [PubMed] [Google Scholar]
- McGaugh, J.L., Cahill, L., Parent, M.B., Mesches, M.H., Coleman-Mesches, K., and Salinas, J.A. 1995. Involvement of the amygdala in the regulation of memory storage. In Plasticity in the central nervous system: Learning and memory (ed. J.L. McGaugh), pp. 17–39. Erlbaum, Mahwah, NJ.
- McKernan, M.G. and Shinnick-Gallagher, P. 1997. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390: 607–611. [DOI] [PubMed] [Google Scholar]
- Milanovic, S., Radulovic, J., Laban, O., Stiedl, O., Henn, F., and Spiess, J. 1998. Production of the Fos protein after contextual fear conditioning of C57BL/6N mice. Brain Res. 784: 37–47. [DOI] [PubMed] [Google Scholar]
- Mineka, S. 1979. The role of fear in theories of avoidance learning, flooding, and extinction. Psych. Bull. 5: 985–1010. [Google Scholar]
- Nikolaev, E., Werka, T., and Kaczmarek, L. 1992. c-Fos protooncogene expression in rat brain after long-term training of two-way active avoidance reaction. Behav. Brain Res. 48: 91–94. [DOI] [PubMed] [Google Scholar]
- Pitkanen, A. and Kemppainen, S. 2002. Comparison of the distribution of calcium-binding proteins and intrinsic connectivity in the lateral nucleus of the rat, monkey, and human amygdala. Pharmacol. Biochem. Behav. 71: 369–377. [DOI] [PubMed] [Google Scholar]
- Pitkanen, A., Savander, V., and LeDoux, J.E. 1997. Organization of intra-amygdaloid circuitries in the rat: An emerging framework for understanding functions of the amygdala. Trends Neurosci. 20: 517–523. [DOI] [PubMed] [Google Scholar]
- Quirk, G.J., Repa, C., and LeDoux, J.E. 1995. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: Parallel recordings in the freely behaving rat. Neuron 15: 1029–1039. [DOI] [PubMed] [Google Scholar]
- Radulovic, J., Kammermeier, J., and Spiess, J. 1998. Relationship between fos production and classical fear conditioning: Effects of novelty, latent inhibition, and unconditioned stimulus preexposure. J. Neurosci. 18: 7452–7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanska, K., Nikolaev, E., Knapska, E., and Kaczmarek, L. 2002. Differential response of two subdivisions of lateral amygdala to aversive conditioning as revealed by c-Fos and P-ERK mapping. NeuroReport 13: 2241–2246. [DOI] [PubMed] [Google Scholar]
- Rodrigues, S.M., Schafe, G.E., and LeDoux J.E. 2001. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J. Neurosci. 21: 6889–6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal, B., Quirarte, G.L., and McGaugh, J.L. 1997. Stress-activated hormonal systems and the regulation of memory storage. Annals NY Acad. Sci. 821: 247–258. [DOI] [PubMed] [Google Scholar]
- Savonenko, A.V. and Zielinski, K. 1998. Effects of conditioned and predictive stimuli on the fly-away escape response in a two-way shuttle box. Acta Neurobiol. Experiment. 58: 321. [DOI] [PubMed] [Google Scholar]
- Savonenko, A., Filipkowski, R.K., Werka, T., Zielinski, K., and Kaczmarek, L.1999a. Defensive conditioning-related functional heterogeneity among nuclei of the rat amygdala revealed by c-Fos mapping. Neuroscience 94: 723–733. [DOI] [PubMed] [Google Scholar]
- Savonenko, A.V., Brush, F.R., and Zielinski, K. 1999b. How do rats cope with the two-way escape problem in a homogeneous shuttle box? Acta Neurobiol. Experiment. 59: 145–157. [DOI] [PubMed] [Google Scholar]
- Savonenko, A.V., Danilets, A.V., and Zielinski, K. 1999c. Studies of individual differences as a method for discriminating the stages of acquisition of a conditioned reflex. Neurosci. Behav. Physiol. 29: 295–303. [DOI] [PubMed] [Google Scholar]
- Schafe, G.E., Atkins, C.M., Swank, M.W., Bauer, E.P., Sweatt, J.D., and LeDoux, J.E. 2000. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J. Neurosci. 20: 8177–8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, D.L. and Davis, M. 2000. Involvement of NMDA receptors within the amygdala in short-versus long-term memory for fear conditioning as assessed with fear-potentiated startle. Behav. Neurosci. 114: 1019–1033. [PubMed] [Google Scholar]
- Werka, T. 1997. The effects of the medial and cortical amygdala lesions on post-stress analgesia in rats. Behav. Brain Res. 86: 59–65. [DOI] [PubMed] [Google Scholar]
- Werka, T. and Zielinski, K. 1998. CS modality transfer of two-way avoidance in rats with central and basolateral amygdala lesions. Behav. Brain Res. 93: 11–24. [DOI] [PubMed] [Google Scholar]
- Wilensky, A.E., Schafe, G.E., and LeDoux, J.E. 2000. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J. Neurosci. 20: 7059–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski, K., Werka, T., and Nikolaev, E. 1993. Latency of the two-way avoidance response in rats: Inhibition of delay. Acta Neurobiol. Experiment. 53: 535–545. [PubMed] [Google Scholar]