Abstract
By a combination of PCR with degenerate primers and low stringency probing, we have isolated a large family of genes related to the Ca2+-sensing receptor from the genome of Fugu rubripes. One of the genes (type I) is the Fugu homologue of the Ca2+-sensing receptor. The remaining genes can be divided into five classes (type II–VI) on the bases of gene structure. In several types, the genes occur in clusters as tandem arrays. These genes appear to be the homologues of the vomeronasal pheromone receptors recently described in rodents. The Fugu genes are expressed in the tissues of the nose, suggesting that they may have a similar physiological role.
Volatile odorants are detected by a large family of G protein-coupled receptors differentially expressed in cells of the olfactory sensory epithelium (1). The detection of pheromones is mediated through neurons of the vomeronasal organ (VNO), and a distinct class of receptors, different from the odorant receptors, was isolated by Dulac and Axel (1995) from a subset of VNO neurons (2). Very recently, three groups (3–5) have characterized a third class of G protein-coupled receptors from a different group of VNO neurons, unrelated to both classes previously found. These G protein-coupled receptors have large extracellular domains and resemble the metabotropic glutamate receptors and the Ca2+-sensing receptor.
In the course of characterizing G protein-coupled receptors in the genome of the puffer fish Fugu rubripes, we encountered members of a large family of receptors related to the Ca2+-sensing receptor, which closely resemble the mammalian pheromone receptors. In this paper, we report on the characterization of these genes and show that they comprise six types, distinguished by sequence homology and gene structure. The genes occur in clusters, and we also show that they are expressed in the nose of the fish, making it likely that they are olfactory detectors.
MATERIALS AND METHODS
Screening of a Fugu Genomic Library with the Bovine Ca2+-Sensing Receptor Gene Probe.
Five segments of the bovine Ca2+-sensing receptor gene (S67307) covering almost the entire coding sequence of the receptor were selected as probes. The probes were prepared by reverse transcriptase-PCR against bovine kidney poly(A)+ RNAs obtained from CLONTECH, and the mixture of probes was labeled with [α-32P]dCTP by random hexamer priming by using the Amersham Redivue kit. Filters containing 160,000 phage plaques from a Fugu genomic DNA library cloned in λ2001 phage were hybridized with the probe mixture in 6X SSC and 0.1% SDS at 55°C for 18 h and washed under low stringent conditions with 1X SSC and 0.1% SDS at 60°C. Signals were detected by the BAS-2000 system (Fuji Photo Film).
Screening Fugu Genomic Libraries for Ca2+-Sensing Receptor-Related Genes.
To find all of the Ca2+-sensing receptor-related genes in the Fugu genome, PCR fragments covering transmembrane (TM) 3 to TM6 of the genes (Ca09, 12, and 13) were labeled with [α-32P]dCTP by random hexamer priming by using Amersham Redivue kit and were used to screen two Fugu genomic DNA libraries, one in λ2001 phage and the other in λDASH phage. Each set of nylon filters contained 160,000 phage plaques. Hybridization was carried as described above.
PCR with Degenerate Primers.
Degenerate primers for PCR were designed from an alignment of the TM regions of the Fugu receptors as well as those of the human (X81086), rat (U20289), and bovine (S67307) Ca2+-sensing receptors. F1 primer (5′-CATCTCNTGYRTYCTGGKNAAAAC-3′), F2 primer (5′-TTYGGNATCASCTTYGTSCTCTG-3′), R1 primer (5′-MAGAAKATSAGCATGCTGAAGGTG-3′), and R2 primer (5′-ACRRTRMAGAAKATSAGCATGCTG-3′) were used for PCR with Fugu genomic DNA. The cycling conditions were 30 s at 95°C, 30 s at 55°C, and 2 min at 72°C for 35 cycles.
F3 primer (5′-TVBTGACCAAGACCAAYCGYATT-3′), F4 primer (5′-TCCTCCAGYAGGGAGCTCTGCTA-3′), and R3 primer (5′-ATGCAGGTDGTGTACATGGTGAA-3′) were designed from an alignment of the TM regions of eight rat metabotropic glutamate receptor (mGluR) genes (X57569, D10891, M92075, M92076, D13963, M92077, D16817, and U63288) and were used for PCR with cycling conditions of 30 s at 95°C, 30 s at 55°C, and 2 min at 72°C for 35 cycles.
Sequencing.
Sequencing was carried out by a combination of the shotgun method, primer walking, and deletion mutant methods by using Dye deoxy terminator chemistry on an Applied Biosystems 373A automated DNA sequencer. Sequence assembly was performed by using Applied Biosystems AutoAssembler and Sequencher software (Gene Codes, Ann Arbor, MI).
Gene Expression.
RNAs were prepared by the acid phenol method by using the ISOGEN kit (Nippon Gene, Toyama, Japan) from brain, nasal tissue, stomatic mucosa, muscle, stomach, intestine, kidney, and liver of adult Fugu. Specific primers for β-actin (6), Ca02.1, Ca07, Ca09, Ca13, and Ca15 were designed as follows: for β-actin, F primer 5′-CAATGGATCCGGTATGTGC-3′, R primer 5′-CGTTGTAGAAGGTGTGATGCC-3′; for Ca02.1, F primer 5′-GACAGCTGTCCTCAAGGAACTCGT-3′, R primer 5′-ACAGGCATTTCTCTCTGGGTTAGG-3′; for Ca07, F primer 5′-CAGAGGTGTCTCCCAGGAACCCAC-3′, R primer 5′-GCACGCATCTCGCTCCTGATTGGA-3′; for Ca09, F primer 5′-GTAAGCTATTTTGCTACATGTTCCTG-3′, R primer 5′-GAACGAACGGGCCACATGGAGCCC-3′; for Ca13, F primer 5′-AGCCACACTTCCTGGTAGTGATGT-3′, R primer 5′-AAGGCTAGGAGGAAACACATACAG-3′; and for Ca15, F primer 5′-CTCTGCTGCTTCGACTGTATCCCC-3′, R primer 5′-CTGTCGAGGAACACAAGCAGTCCG-3′.
The cycling condition was 30 s at 95°C, 30 s at 55°C, and 2 min at 72°C for 30 cycles.
Phylogenetic Analysis.
The inferred amino acid sequences of TM regions of human, rat, bovine Ca2+-sensing receptors, rat mGluRs, rat vomeronasal pheromone receptors (AF016178, AF016180, AF016181, AF016182, AF016186, and those in ref. 5), mouse vomeronasal pheromone receptors (AF011411, AF011412, AF011413, AF011414, AF011415, and those in ref. 5) and Fugu Ca2+-sensing receptor-related genes (AB008857–AB008862 and AB009032–AB009044) were aligned by using the sinca program (Fujitsu Limited, Chiba, Japan). The phylogenetic tree was constructed by using the Neighbor Joining method (7) with bootstrap analysis.
RESULTS
The initial experiments were directed toward the isolation of the Fugu Ca2+-sensing receptors. Five probes were prepared that covered almost the entire length of the bovine Ca2+-sensing receptor and were used to screen a genomic library made in bacteriophage λ. Eighteen positive clones were found among 160,000 plaques probed (≈6 × genome equivalents). These clones were classified into four different groups by a fingerprinting method modified for fluorescent labeling. Shotgun clones of representative phage of each group were prepared and screened with a probe covering the TM sequence of the bovine receptor, and positive clones were sequenced. We obtained seven different TM sequences with homology to the Ca2+-sensing receptors. This result already showed that the genes were clustered because more than one gene was present on some of the phages. These seven sequences were aligned together with bovine, rat, and human Ca2+-sensing receptor TM sequences, and several highly conserved segments were identified. Degenerate primers were designed, and PCR was performed on Fugu genomic DNA. The products were cloned and sequenced, and additional seven new TM sequences were found. The total of 14 sequences was classified into five groups depending on sequence similarity. To identify all of the Ca2+-sensing receptor-related genes in the Fugu genome, we screened the Fugu genomic library at low stringency with a probe of the mixture of three fragments obtained by PCR with the degenerate primers. We found 117 clones from 320,000 plaques. These were now screened by PCR by using specific primers corresponding to the 14 TM sequences previously found and were classified roughly into groups. A representative of each group was shotgun sequenced, and it immediately became clear that many of the phages contained multiple genes, indicating that the genes occur in clusters of closely related sequences in the genome. A representative of a sixth class (Ca15) was found while characterizing these phages.
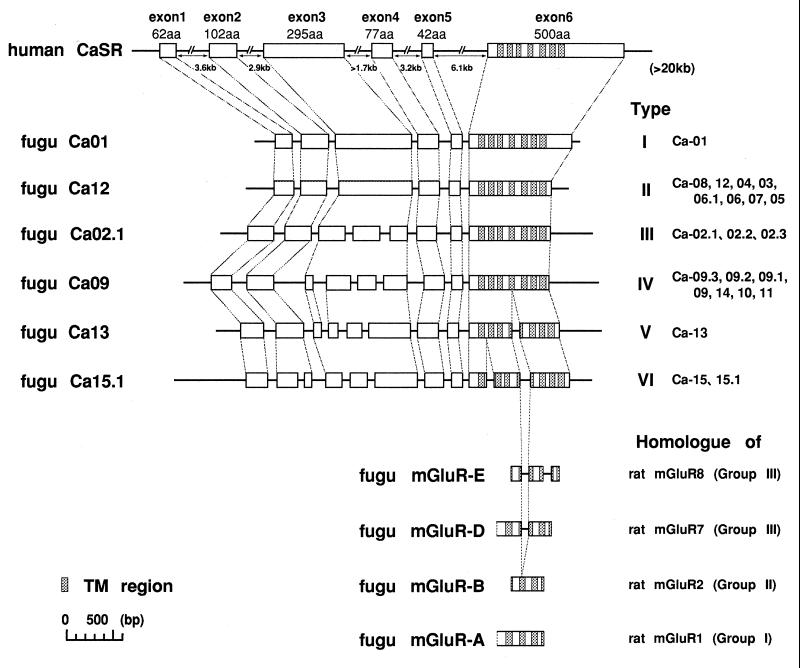
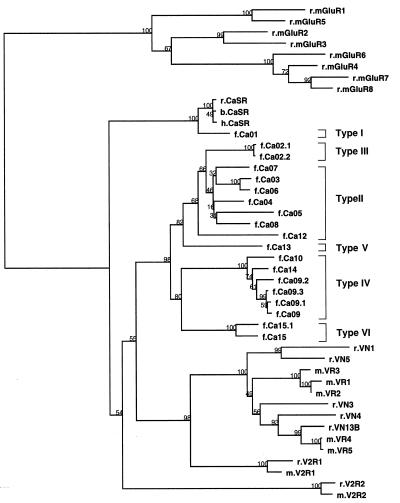
One member of each type was sequenced completely, and the results are shown in Fig. 1. Fig. 1 also shows the gene structure of the human Ca2+-sensing receptor as well as the sequence of fragments of four metabotropic glutamate receptors cloned from the Fugu genome by PCR with degenerate primers. The relationship between the inferred amino acid sequences is shown in Fig. 2, in the form of a phylogenetic tree, which includes the related metabotropic glutamate receptors and the mammalian VNO pheromone receptors. Fugu Ca01 is identified as the Ca2+-sensing receptor because it responds to Ca2+ stimuli (data not shown). The structure of the gene is identical to that of its human counterpart except that the Fugu gene is compact, ≈4 kb in length, whereas the human gene with much larger introns is more than five times larger. The amino acid sequence of the Fugu Ca2+-sensing receptor is very similar to that of the mammalian receptors.
Figure 1.
Gene structure of the Ca2+-sensing receptor and related genes. Gene structure of the human gene is drawn on the basis of the data (8). Boxes of exon 1 and exon 6 show coding regions only. The introns of the human gene are shortened, but the Fugu genes are to scale. The Ca01 (type I) gene is the Fugu Ca2+-sensing receptor gene. Also shown are fragments of four metabotropic glutamate receptor genes cloned from Fugu by degenerate primer PCR. Where connecting lines are drawn to show the position of initiation codon, boundaries of introns, and the position of stop codon, this implies that they are in exactly the same position in the different genes. The shaded regions are TM sequences. All sequences of Fugu genes are deposited in the DNA Database in Japan (accession nos. AB008857–AB008866).
Figure 2.
Phylogenetic tree of the TM regions of the Ca2+-sensing-related receptors. Bootstrap values are shown at the branch points. The Fugu receptors are related closely to the mammalian vomeronasal receptors (VNs, VRs, V2Rs); both are related to the Ca2+-sensing receptor (CaSR) and more distantly to the metabotropic glutamate receptors (mGluRs). The first small letter of each gene shows species: r, rat; m, mouse; h, human; b, bovine; f, Fugu.
The remaining five classes of receptors are all related to each other to varying extents and to the Ca2+-sensing receptors. Fig. 2 shows their relationship to the metabotropic glutamate receptors and to the VNO pheromone receptors.
The gene structure of type II receptors is identical to that of the Ca2+-sensing receptor. Type III and IV receptor genes have extra introns in the extracellular domain, and type V and VI receptors have extra introns in the TM domain as well as other differences in the extracellular domain. The common intron in the TM domain of types V and VI also is found in two of the Fugu metabotropic glutamate receptor genes.
Further characterization of the complete structure of the clusters is being undertaken, but we have shown by sequencing a number of phages that Ca08, 12, 04, and 03 are linked in a tandem array, as are Ca09.3, 09.2, 09.1, and 09. Some of the genes, such as Ca09 and 09.1, have very similar amino acid sequences, suggesting that the duplication may be relatively recent. We also have found some pseudogenes, that is, genes with stop codons in the coding region or genes with deletions and inversions.
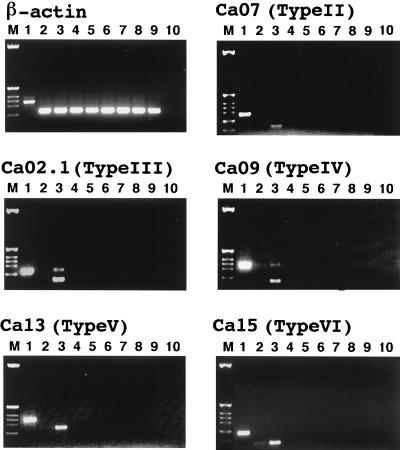
The expression of these putative pheromone genes was analyzed in different Fugu tissues by PCR on cDNA preparations by using specific primers for each of the receptor types. PCR primers used can distinguish between gene types but in some cases cannot distinguish between the members in the same type because of close resemblance in sequence. The results are shown in Fig. 3. All of the five types examined express specifically in tissues of the nose. Ca15 shows a small amount of expression in the brain. There also seems to be some unspliced messenger synthesis in Ca02.1 and Ca09, and the latter also shows this in brain. Experiments, not shown here, demonstrate that the tissue pattern of the Ca2+-sensing receptor is different, with most abundant expression in brain and kidney.
Figure 3.
Expression of Ca2+-sensing receptor related genes in different tissues of Fugu. Primers were deduced for each of genes such that the fragment included an intron. mRNA preparations were reverse transcribed, and PCR was performed as designed in Materials and Methods. Lane 1 contains genomic DNA; lane 10 is another control in which reverse transcriptase was omitted. The other lanes contain mRNA from the following Fugu organs: 2, brain; 3, nose; 4, stomatic mucosa; 5, muscle; 6, stomach; 7, intestine; 8, kidney; and 9, liver. The marker used was the HinfI-digested pBR322.
DISCUSSION
Analysis of the compact genome of Fugu rubripes reveals a large family of receptors closely related to the Ca2+-sensing receptor. These appear to be the fish homologues of the vomeronasal receptors recently reported in mice and rats (3–5). Matsunami and Buck (4) report that they found related families of genes in Xenopus laevis; our results show that this can now be extended to fish. We also have shown that the genes are expressed specifically in the tissues of the nose, which suggests that they may have an olfactory function and, based on homology, are putative pheromone receptors.
Our analysis of the genomic structures of the Fugu genes shows that they fall into five classes. One of the classes has an intron structure identical with that of the Ca2+-sensing receptor, but the other four classes are different. An evolutionary connection to the metabotropic glutamate receptor genes is confirmed by finding an intron in the TM region common to some members of each family of genes. Because ligand specificity of these receptors resides in the extracellular domain, the different types of genes may correspond to different classes of ligands but, at the moment, we do not know what these may be, although there is a report that tetrodotoxin may be a pheromone for Fugu (9).
Matsunami and Buck (4) estimated that there were ≈140 members of this family of receptors in the mouse genome as assayed by hybridization experiments. We can estimate the number of receptor genes in Fugu by comparing the yields of the different classes in cloning experiments. In one experiment, we obtained 74 phages, of which 9 were isolates of type V receptors, which are not clustered. We estimate that this library contained six genome equivalents, and preliminary sequencing results indicate that, on the average, three genes from the clusters are found on each phage. The clusters would therefore account for 33 genes per genome, and our estimate for the number of genes is 30–40, approximately one-quarter of the number estimated in the mouse. However, the number of functional genes may be less because we have found pseudogenes, that is, genes with stop codons in their coding sequences and genes with deletions and inversions, as in the rodent case.
The elegant work on the odorant and pheromone receptors shows that the discovery of genes expressed in a few cells by cDNA cloning depends on the analysis of single cells. The compact Fugu genome lends itself to exhaustive analysis of gene families and allows gene discovery by homology alone. Thus, the two approaches are complementary, but validating that these receptors actually mediate the effects of pheromones will require physiological experiments.
Acknowledgments
We thank Kaori Nagao, Yuko Inazu, and Masahiro Furuno of the Pharmaceutical Frontier Research Laboratories of Japan Tobacco, Inc., for help with data analysis and for valuable discussion.
ABBREVIATIONS
- VNO
vomeronasal organ
- TM
transmembrane
- mGluR
metabotropic glutamate receptor
Footnotes
References
- 1.Buck L, Axel R. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 2.Dulac C, Axel R. Cell. 1995;83:195–206. doi: 10.1016/0092-8674(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 3.Herrada G, Dulac C. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- 4.Matsunami H, Buck L B. Cell. 1997;90:775–784. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- 5.Ryba N J P, Tirindelli R. Neuron. 1997;19:371–379. doi: 10.1016/s0896-6273(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 6.Venkatesh B, Tay B H, Elgar G, Brenner S. J Mol Biol. 1996;259:655–665. doi: 10.1006/jmbi.1996.0347. [DOI] [PubMed] [Google Scholar]
- 7.Saitou N, Nei M. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 8.Pollak M R, Brown E M, Chou Y-H W, Hebert S C, Marx S J, Steinmann B, Levi T, Seidman C E, Seidman J G. Cell. 1993;75:1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura K. Nature (London) 1995;378:563–564. doi: 10.1038/378563b0. [DOI] [PubMed] [Google Scholar]