Abstract
Transforming growth factor-β (TGF-β) is a multifunctional cytokine signaling to the nucleus through cell surface transmembrane receptor serine/threonine kinases and cytoplasmic effectors, including Smad proteins. We describe a novel modulator of this pathway, TLP (TRAP-1-like protein), which is 25% identical to the previously described Smad4 chaperone, TRAP-1, and shows identical expression patterns in human tissues. Endogenous TLP associates with both active and kinase-deficient TGF-β and activin type II receptors, but interacts with the common-mediator Smad4 only in the presence of TGF-β/activin signaling. Overexpression of TLP represses the ability of TGF-β to induce transcription from SBE-Luc, a Smad3/4-specific reporter, while it potentiates transcription from ARE-Luc, a Smad2/4-specific reporter. Consistent with this, TLP inhibits the formation of Smad3/4 complexes in the absence of effects on phosphorylation of Smad3, while it affects neither Smad2 phosphorylation nor hetero-oligomerization. We propose that TLP might regulate the balance of Smad2 and Smad3 signaling by localizing Smad4 intracellularly, thus contributing to cellular specificity of TGF-β transcriptional responses in both normal and pathophysiology.
Keywords: Smad proteins/TGF-β signaling/TRAP-1/TGF-β receptors
Introduction
Ligands of the transforming growth factor-β (TGF-β) superfamily and their downstream signal transduction components regulate a variety of physiologic and pathologic processes, such as embryogenesis, wound healing, fibrosis and immunomodulation, and are involved in growth control, oncogenesis and heritable disorders (Roberts and Sporn, 1990; de Caestecker et al., 2000; Massague et al., 2000).
Binding of TGF-β to type II receptors (TβRII) leads to recruitment and transphosphorylation of type I receptors (TβRI). The activated TβRI phosphorylates receptor-activated Smad proteins, Smad2 and Smad3, which then dissociate from the receptor, hetero-oligomerize with a common partner Smad4 and translocate to the nucleus, where they regulate the transcription of target genes (Massague and Chen, 2000; Miyazono et al., 2000). While Smad2 and Smad3 appear to be phosphorylated synchronously in cells, they activate unique transcriptional targets, based in part on their differential DNA binding activity. Thus Smad3 binds cognate GTCT motifs directly, while an insertion in the N-terminal MH1 domain of Smad2 precludes its direct DNA binding, permitting only indirect regulation of gene transcription via binding to transcription factors (Yagi et al., 1999). These different roles of Smad2 and Smad3 are most evident in effects of their targeted deletion, which result in embryonic lethality and an adult phenotype, respectively (Weinstein et al., 2000). Whether the balance of signaling flux through Smad2 and Smad3 is regulated in cells independent of their relative levels of expression is not known.
Among a group of potential modulators of Smad signaling are a number of non-Smad proteins, including Daxx (Perlman et al., 2001), SARA (Smad anchor for receptor activation) (Tsukazaki et al., 1998), Hgs/Hrs (hepatic growth factor-regulated tyrosine kinase substrate) (Miura et al., 2000), STRAP (Datta and Moses, 2000), FKBP12 (Wang et al., 1996), TRIP-1 (Chen et al., 1995), the Bα subunit of phosphatase 2A (Griswold-Prenner et al., 1998), Disabled-2 (Dab2) (Hocevar et al., 2001) and TGF-β receptor I-associated protein-1 (TRAP-1) (Charng et al., 1998), all of which have been identified as interactors of TβRI and/or TβRII. Among these, SARA and Hrs have been shown to facilitate Smad2/3 recruitment to the activated TβRI/ActRI (Tsukazaki et al., 1998; Miura et al., 2000) and the adaptor molecule Dab2, which binds TβRs, Smad2 and Smad3, has been suggested to bridge the TGF-β receptor complex to the Smad pathway (Hocevar et al., 2001). STRAP was shown to stabilize the association between Smad7 and the activated receptor, thus blocking Smad2/3-mediated transcriptional activation (Datta and Moses, 2000).
We have studied one of these receptor interacting proteins, TRAP-1, and shown that it binds selectively to inactive TGF-β and activin receptor complexes, forming a transient complex with Smad4 when signaling was activated and potentially facilitating transfer of Smad4 to the acceptor, Smad2/3 (Wurthner et al., 2001). To identify homologs and orthologs of TRAP-1, we searched the public databases at NCBI. In this study, we report the identification and characterization of a novel human cytoplasmic protein, which we named TRAP-1-like protein (TLP), that shows 25% identity to TRAP-1, shares a similar overall domain organization and parallels the tissue distribution of TRAP-1 in human organs. We show that TLP interacts predominantly with TβRII in vivo and colocalizes with the receptor complex in a signal-independent way in submembrane vesicular domains. Like TRAP-1, TLP associates with Smad4 only in the presence of signal propagation. While TLP has no effect on phosphorylation of Smad2 or Smad3, it differentially regulates TGF-β-induced gene expression by activating Smad2-dependent responses and blocking Smad3-dependent transcription through selective inhibition of Smad3/4 complex formation. These observations support the hypothesis that TLP is a modulator of the TGF-β response that functions as an adaptor protein, coupling the TGF-β receptor complex to the Smad pathway, with the unique role of regulating the balance of Smad3 versus Smad2 signaling.
Results
Cloning and domain organization of the full-length human TLP shows homology with TRAP-1
To assess whether TRAP-1 belongs to a family of proteins interacting with the TGF-β receptor superfamily, we searched the DNA database and identified a partial cDNA clone of unknown function, KIAA0770 (DDBJ/EMBL/GenBank accession Number AB018313; gi:20521649), which encodes 731 amino acids and shows 25% identity and 44% similarity to TRAP-1 in a sequence comparison with BLAST at NCBI (Altschul et al., 1997). The sequence of the 5′ end of KIAA0770 was cloned from cDNAs prepared from HepG2 and human liver mRNA by 5′RACE-PCR. An in-frame STOP codon was detected 106 bp upstream of the ATG start site. The full-length cDNA of KIAA0770 encodes a protein of 875 amino acids and predicted molecular mass of ∼100 kDa, which we designated TLP (Figure 1A). The expression pattern of TLP mRNA was examined by human multitissue northern blot using a 719 bp fragment at the 3′ end of TLP, which showed no significant similarity to TRAP-1. One major band of approximately 5.5 kb was detected in all adult tissues examined, similar to TRAP-1 for which two transcripts of 4.5 and 6.5 kb are known (Charng et al., 1998) (Figure 2A). TLP is ubiquitously expressed, with higher levels of message detected in brain, heart, skeletal muscle, kidney and spleen. In peripheral blood leukocytes, an extra 5.8 kb band was observed. Analysis of expression in a variety of human cell lines showed some differences between expression of TLP and TRAP-1, with the highest levels of expression of TLP expressed in the neuronal cell line SKN-SH, consistent with the original cloning of KIAA0770 in a brain cDNA library (Figure 2B).
Fig. 1. Sequence similarity and domain organization of human TLP and TRAP-1. (A) Alignment of TRAP-1 and TLP amino acid sequence. Identical and homologous residues are shaded black and gray, respectively. Numbers represent amino acid positions. Blue and red lines denote the hypothetical CNH and CLH domains respectively. (B) Domain organization of human TLP and TRAP-1: blue box, CNH domain; red box, CLH domain. Numbers represent amino acid residue positions.
Fig. 2. TLP and TRAP-1 show similar tissue distribution. (A) TLP and TRAP-1 are ubiquitously expressed in similar patterns. A single TLP transcript of approximately 5.5 kb was detected in all human tissues examined, with the exception of peripheral blood leukocytes which displayed two distinct transcripts of 5.5 and 5.8 kb. (B) TLP and TRAP-1 expression in human cell lines: HepG2, hepatocarcinoma; Colo357, pancreatic carcinoma; MDA468, breast carcinoma; BxPC3, pancreatic carcinoma; HeLa, cervix adenocarcinoma; SKN-SH, neuroblastoma.
A subsequent domain search for protein motifs using SMART (Schultz et al., 2000) showed an N-terminal Citron homology (CNH) domain between amino acids 10 and 289, and a Clathrin heavy-chain repeat homology (CLH) domain between amino acids 404 and 548. CNH, which is more stringently conserved in TLP than in TRAP-1, is a recently described domain found in a number of signaling molecules, such as the GDP/GTP exchange proteins for Rho1, ROM1 and ROM2 in Saccharomyces cerevisiae, and the RhoGTP/RacGTP-binding protein, Citron, in Mus musculus (Madaule et al., 1995). TLP also contains a CLH motif similar to seven such motifs present in the filamentous leg of clathrin, a protein that polymerizes onto the cytoplasmic surface of protein-coated membrane vesicles (Ybe et al., 1999). These motifs have been suggested to mediate protein–protein interactions or, alternatively, to represent a clathrin-binding domain (Nakamura et al., 1997). In work published while this study was in progress, a human homolog of the S.cerevisiae vacuolar protein sorting gene product Vam6p/Vps39p was cloned from a brain cDNA library that is identical with TLP except for an 11 amino acid insertion between Val46 and Ser47 in Vam6p/Vps39p (Caplan et al., 2001). Overexpression of hVam6p/Vps39p resulted in clustering and fusion of lysosomes and late endosomes, and suggested that the protein might function as a tethering/docking factor.
TLP associates with TGF-β and activin receptors constitutively
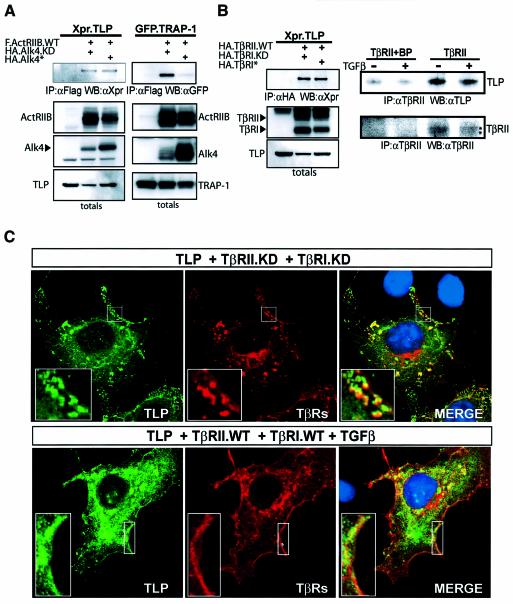
To test whether TLP, like TRAP-1, might also interact with receptors of the TGF-β superfamily, COS-1 cells were co-transfected with TLP and TβRs either in their wild-type (WT), kinase-deficient (KD) or constitutively activated (asterisk) forms. Immunoprecipitation of receptors followed by western blotting for TLP showed that TLP coprecipitates with TβRII, and with a markedly lower affinity for TβRI (Figure 3A, left panel), similarly to TRAP-1 (Figure 3A, rightmost panel). TLP binds the WT and KD forms of TβRII equally well (Figure 3A, lanes 3–4), unlike TRAP-1 which interacts more strongly with KD TβRII (Figure 3A, right panel).
Fig. 3. TLP interacts with TGF-β and activin receptors in vivo. (A) TLP and TRAP-1 display different binding affinities for TβRs. COS-1 cells were transiently transfected with Xpr.TLP (left panels) or GFP.TRAP-1 (right panels) in combination with the indicated HA-TβRs. Thirty hours after transfection, cells were serum starved for 12 h and cell lysates were immunoprecipitated with anti-HA antibody and blotted as indicated. Lysates of cells untransfected or transfected with TLP or TRAP-1 cDNA alone were analyzed as negative controls. Top panels, immunoprecipitated complexes; bottom panels, unfractionated extracts showing expression levels of the transfected proteins. (B) TLP associates with ActRs but not with BMPRII. COS-1 cells were transiently transfected with Xpr.TLP and Flag.ActRIIB or BMPRII receptors as indicated. Lysates were subjected to immunoprecipitation with anti-Flag and blotted with anti-Xpr antibody. In the two leftmost lanes, lysates of cells untransfected or transfected with TLP cDNA alone were analyzed as negative controls. Expression levels of receptors and TLP were confirmed by western blot analysis of total cell extracts using anti-Xpr and anti-Flag antibodies, respectively. WT, wildtype; KD, kinase-deficient (K277R) mutant; asterisk, mutationally activated (T204D) mutant.
To examine whether TLP interacts with other type II receptors of the TGF-β superfamily, COS-1 cells were co-transfected with TLP and activin receptor type IIB (ActRIIB) or bone morphogenetic protein receptor type II (BMPRII). Figure 3B shows that TLP interacted with ActRIIB but not BMPRII, despite the efficient expression of the latter receptor in COS-1 cells.
We next asked whether the binding of TLP to TGF-β/activin receptors is dependent on the activation state of the signaling pathway. Since TβRs are usually expressed at low levels on the cell surface, a commonly used approach is to overexpress WT TβRII and TβRI and stimulate with TGF-β. However, we previously showed that overexpression of WT TβRII in COS-1 cells results in ligand-independent activation of the TGF-β pathway (Wurthner et al., 2001). Based on this, we co-transfected COS-1 cells with TLP, WT TβRII and TβRI made either kinase deficient or constitutively active by a specific point mutation (Wieser et al., 1995; Weis-Garcia and Massague, 1996). Immunoprecipitation of receptor complexes followed by western blotting for TLP revealed no differences in association, indicating that TLP is constitutively found in association with the activin (Figure 4A, left panel) and TGF-β receptor complexes (Figure 4B, left panel). When TRAP-1 binding to activin receptors was tested under the same conditions, the receptor association was abolished in cells with active signal propagation, consistent with previous findings (Wurthner et al., 2001).
Fig. 4. TLP interacts with TGF-β/activin receptor complexes in a signal-independent fashion. (A) In contrast to TRAP-1, TLP associates with ActRIIB constitutively. COS-1 cells were transiently transfected with Xpr.TLP (left panel) or with GFP.TRAP-1 (right panel) in combination with the indicated epitope-tagged activin receptors type II and type I. Lysates were immunoprecipitated with anti-Flag and blotted as indicated. (B) TLP associates with TGF-β receptor complex constitutively. Left panels: COS-1 cells transfected with Xpr.TLP and the indicated HA-TβRs type II and type I were immuno precipitated with anti-HA and blotted with anti-Xpr. Bottom left panels: control western blots for the immunoprecipitations. Leftmost lane (A and B): lysate of cells transfected with either TLP or TRAP-1 cDNA alone, negative control. Right panels: endogenous TLP associates with TβRII constitutively. HaCaT cells treated with TGF-β for 1 h, followed by treatment with cross-linker for 20 min, were immunoprecipitated with anti-TβRII and blotted with anti-TLP specific rabbit polyclonal antibody (749). Comparable levels of TβRII in the immunoprecipitates are shown. Leftmost lanes: lysate of cells untreated (–) or treated (+) was immunoprecipitated in the presence of TβRII specific blocking peptide (sc-400P) as negative control. (C) TLP colocalizes with TGF-β receptor complex at the plasma membrane and submembrane vesicular domains in a ligand-independent fashion. COS-1 cells were transiently transfected with Xpr.TLP and either HA.TβRII.KD and HA.TβRI.KD in the absence of TGF-β (top panels) or HA.TβRII.WT and HA.TβRI.WT, and treated with TGF-β for 20 min (bottom panels). TLP and TβRs were visualized by the monoclonal anti-Xpr antibody (green) and by the polyclonal anti-HA antibody (red), respectively. DAPI staining (blue) highlights the location of nuclei. Colocalization of TLP and TβRs appears as yellow. Areas marked by a rectangle are enlarged and shown as insets.
We next investigated whether endogenous TLP is found in association with endogenous TGF-β receptors in vivo and whether TGF-β treatment affected this interaction. To assess this, we raised polyclonal antibodies to peptides of TLP which share no homology with TRAP-1 and that specifically detect endogenous TLP in immunoprecipitation and immunoblotting (Supplementary figure S1 available at The EMBO Journal Online). HaCaT cells were either left untreated or stimulated with TGF-β for 1 h followed by cross-linking, immunoprecipitation of endogenous TβRII and immunoblotting of endogenous TLP (Figure 4B, right panels). Consistent with the overexpression data, endogenous TLP coprecipitated with TβRII in a TGF-β-independent manner. Thus, TLP is part of a multiprotein signaling complex that contains TGF-β receptors. Together, these data show that TLP interacts specifically with TGF-β/activin and not with bone morphogenetic protein (BMP) receptors in vivo and that, unlike TRAP-1, TLP remains bound to actively signaling receptor complexes.
Next, we assessed the intracellular localization of epitope-tagged TLP and TβRs in intact cells in either the absence or the presence of active signaling by indirect immunofluorescence and confocal microscopy (Figure 4C). Consistent with a possible localization on intracellular vesicles, TLP displayed a punctate staining pattern that was present throughout the cytoplasm and was excluded from the nucleus. TβRs were detected at the plasma membrane and accumulated in cytoplasmic punctate vesicular structures, consistent with their presence on both endosomes and clathrin-negative endocytic compartments (Doré et al., 1998; Yao et al., 2002; Di Guglielmo et al., 2003). Co-transfection of TLP and TβRs at low to moderate levels showed extensive, although not complete, colocalization of the two staining patterns in cytoplasmic punctate vesicular structures and more prominently in cortical vesicles (Figure 4C, top panels). While TGF-β treatment was found to cause a partial redistribution of both TLP and TβRs from punctate vesicular structures to the cytosol, TLP and TβRs remained colocalized. Together with our biochemical analysis, these results demonstrate that TLP and TβRs colocalize to common subcellular domains independently of signaling and that their association is not disrupted upon ligand stimulation.
TLP associates with Smad4 in a TGF-β-dependent fashion
Since previous work from our laboratory suggested that TRAP-1 functions as a chaperone for Smad4 (Wurthner et al., 2001), we investigated whether TLP might also interact with Smad4 in vivo. Transfection of COS-1 cells with Smad4 and TLP in either the absence or the presence of constitutively active TβRI showed that TLP associates with Smad4 in the absence of TGF-β signaling, while this association is attenuated 48 h after transfection of constitutively active TβRI (Figure 5A). TLP did not associate with Smad2 or Smad3 in either the presence or the absence of signaling (data not shown). Overexpression of Smad2 does not affect the association of TLP with Smad4 in the absence of signaling, but promotes dissociation of TLP from Smad4 when signaling is activated (Figure 5A). This observation is consistent with the hypothesis that Smad4 has a higher affinity for phosphorylated Smad2 than for TLP and suggests that, whereas TLP might localize Smad4 to receptor complexes, TLP and Smad2 form mutually exclusive complexes with Smad4. Under the same experimental conditions, TRAP-1 binds Smad4 only when signaling is activated, and this association is inhibited by overexpression of phosphorylated Smad2, consistent with our previous findings (Figure 5B). The opposite dynamics of association with Smad4 displayed by TLP and TRAP-1 suggest that these homologous receptor interactors might have different roles in signal propagation from TGF-β/activin receptors.
Fig. 5. TLP associates with Smad4 in vivo. (A) TLP and Smad2 form mutually exclusive complexes with Smad4. COS-1 cells that co- expressed myc.Smad4 and Xpr.TLP were transfected with or without HA.TβRI* to activate TGF-β pathway in either the absence or the presence of Flag.Smad2. Lysates were immunoprecipitated with myc and probed with Xpr antibody. (B) Effect of TGF-β signaling and Smad2 expression on TRAP-1/Smad4 association. COS-1 cells that co-expressed myc.Smad4 with Flag.TRAP-1 were transfected with or without HA.TβRI* in either the absence or the presence of Flag.Smad2 as competitor. Cells treated as in (A) were immunoprecipitated with myc and probed with Flag antibody. Leftmost lane: lysate of cells transfected with (A) TLP cDNA or (B) TRAP-1 cDNA alone was analyzed as negative control. (C) TGF-β-dependent interaction between endogenous TLP and Smad4. Lysates of HaCaT cells untreated (–) or treated (+) with TGF-β for 1 h were subjected to Smad4 immunoprecipitation and immunoblotting with a TLP antiserum (749) (top panel). Equal levels of Smad4 in the immunoprecipitates are shown (bottom panels). Two leftmost lanes: lysates immunoprecipitated with an irrelevant IgG were analyzed as negative control.
Contrasting with this pattern, endogenous TLP and Smad4 associate only after treatment with TGF-β for 1 h (Figure 5C). This ligand-dependent formation of TLP/Smad4 complexes has been confirmed using a reciprocal immunoprecipitation–western blotting design (data not shown). The lack of detectable association of endogenous TLP and Smad4 in the absence of ligand suggests that overexpression may lead to aberrant association of TLP and Smad4 in non-physiological compartments. Again, no association of endogenous Smad2 or Smad3 with endogenous TLP was found in either the presence or the absence of ligand (data not shown).
TLP inhibits Smad3- and activates Smad2-dependent transcription in TGF-β/activin reporter assays but has no effect on BMP-dependent transcription
To explore the functional significance of the association of TLP with TGF-β/activin receptors and with Smad4, we tested the effects of TLP on transcription from TGF-β/activin-responsive promoters. Using the artificial SBE4-Luc reporter, which contains four tandem repeats of Smad-binding elements (Zawel et al., 1998) and measures a Smad3/4-specific response, we showed that overexpression of TLP suppressed the TGF-β-induced transcriptional activity in a dose-dependent manner (Figure 6A). Basal transcription from the SBE4-Luc promoter was also suppressed by TLP (Figure 6A, open bars), suggesting autocrine TGF-β signaling in Hep3B cells. Similar results were found in HepG2 and HEK 293 cells (data not shown). Surprisingly, when a wider range of concentrations of TLP (0.06–0.6 µg) was tested, we found that TLP had a dose-dependent biphasic effect on TGF-β-dependent transcription (data not shown). The selective response of the SBE4-Luc promoter to Smad3, but not Smad2, was confirmed in Hep3B cells (Figure 6B), consistent with the inability of Smad2 to bind to SBE sites (Yagi et al., 1999).
Fig. 6. TLP inhibits Smad-3 and activates Smad-2 dependent transcription in TGF-β/activin reporter assays while having no effect on BMP signaling. (A and C) Effect of TLP on TGF-β-induced transcription of SBE4 and ARE reporters. Hep3B cells were transiently transfected with a control vector or increasing amounts of TLP along with either (A) SBE4 or (C) ARE reporter and FAST1. (B and D) Effect of Smad2 and Smad3 on TGF-β-induced transcription of SBE4 and ARE. Hep3B cells were transiently transfected with a control vector or with Smad2- and Smad3-expressing vectors as indicated, along with either (B) SBE4 or (D) ARE reporter and FAST1. Luciferase activity in cells treated with 5 ng/ml TGF-β1 (black bars) or left untreated (open bars) is shown. (E–G) TLP blocks both TGF-β and activin-dependent Smad3-specific transcription while having no effect on activation of the BMP-specific BRE reporter. (E and F) The indicated expression vectors were cotransfected with BRE reporter and Hep3B cells were treated with 100 ng/ml BMP2 (black bars) or left untreated (open bars). (G) Hep3B cells were transiently transfected with a control vector or TLP (0.2 µg/well) along with SBE4. The cells were treated with 5 ng/ml TGF-β1 or 25 ng/ml activin A (black bars) or left untreated (open bars). (H) TLP blocks TGF-β-mediated transcription of human Smad7 promoter. The Smad7 promoter construct was cotransfected with increasing amounts of TLP in Hep3B cells treated with 5 ng/ml TGF-β (black bars) or left untreated (open bars). Luciferase activity was normalized to β-galactosidase activity and plotted as mean ± SD of triplicates from a representative experiment.
To address the effect of TLP on a Smad2-dependent promoter, we examined induction of the activin response element (ARE) from the Xenopus Mix.2 gene (Labbé et al., 1998). Co-transfection of Hep3B or HepG2 cells (data not shown) with the ARE-Luc reporter FAST1 and with increasing amounts of TLP showed that TLP enhanced both basal and TGF-β-induced activity of ARE-Luc 2- to 4-fold (Figure 6C), resembling the response to overexpressed Smad2 and Smad4 (Griswold-Prenner et al., 1998) (Figure 6D). Since Smad2 and Smad3 have opposite activities on the ARE reporter (Figure 6D), as reported previously (Nagarajan et al., 1999; Piek et al., 2001), activation of the ARE reporter by TLP can be attributed to a positive effect of TLP on flux through the Smad2 pathway (ARE activator) and/or a negative effect of TLP on signaling through the Smad3 pathway (ARE inhibitor).
To see if the transcriptional effects of TLP mimicked its pattern of receptor interaction, we tested both activin- and BMP-dependent signaling in Hep3B cells. Overexpression of TLP interfered with activin-A-induced transcription from the SBE4 reporter (Figure 6G, right panel) but had no effect on the Smad1-dependent BRE4-Luc reporter (Korchynskyi and ten Dijke, 2002) (Figure 6E and F). Together, the data suggest that although TLP associates with the common mediator Smad4, it does not affect Smad4 transcriptional function generally but, rather, is likely to couple Smad4 with Smad2/3 signaling by recruiting Smad4 to TGF-β/activin receptor complexes.
To confirm that TGF-β-induced transactivation of endogenous Smad3-target promoters is also inhibited by TLP, we examined transcription of the human Smad7 promoter Smad7-Luc, in which a single Smad3/4 binding element is embedded in the context of a 4.6 kbp native chromatin region (von Gersdorff et al., 2000). TLP decreased TGF-β-induced activity of the Smad7 promoter in a dose-dependent manner, similarly to its effects on SBE4-Luc (Figure 6H), demonstrating that it also negatively impacts TGF-β signaling through the Smad3/4 pathway to physiological target promoters in the cell.
TLP does not affect the extent or the kinetics of TGF-β-induced Smad2 or Smad3 phosphorylation
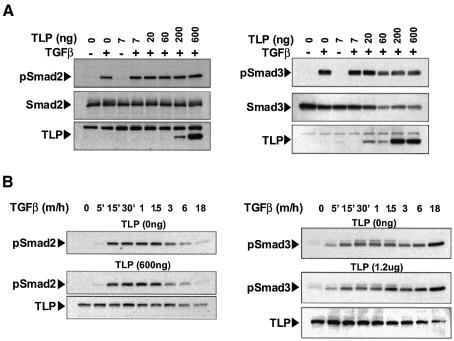
Since the transcriptional activity of both Smad2 and Smad3 is dependent on their phosphorylation by activated TβRI (Macias-Silva et al., 1996), we examined whether TGF-β-mediated phosphorylation of Smad2/3 is regulated by TLP. As seen in Figure 7A, increasing doses of TLP had no effect on phosphorylation of overexpressed Smad2 (left panels) or Smad3 (right panels) in Hep3B cells stimulated with TGF-β1 for 30 min. The decrease in pSmad3 levels in the presence of the highest concentrations of TLP correlates with reduced levels of total Smad3 and it is likely due to promoter interference between TLP and Smad3 expression vectors. A time course of phosphorylation of endogenous Smad2 and Smad3 in Hep3B cells transfected either with vector control or TLP is shown in Figure 7B. Whereas Smad2 phosphorylation (left panels) was more transient than that of Smad3 (right panels), neither the intensity nor the onset and duration of Smad2 and Smad3 activation were affected by high levels of TLP. Together, these results demonstrate that levels of TLP which, in the same experimental setting, potently inhibited the Smad3-dependent response and enhanced the Smad2-dependent response do not affect the extent and kinetics of phosphorylation of either endogenous or overexpressed Smad2 and Smad3.
Fig. 7. TLP has no effect on TGF-β-induced phosphorylation of Smad2 and Smad3. (A) Increasing concentrations of TLP have no effect on the extent of Smad2 and Smad3 phosphorylation induced by 30 min stimulation with TGF-β. Hep3B cells were transiently transfected with increasing amounts of Xpr.TLP (7–600 ng/well) along with either Flag.Smad2 (left panel) or Flag.Smad3 (right panel). After 18 h of serum starvation, cells were treated with 5 ng/ml TGF–β1 for 30 min (+) or left untreated (–). Smad phosphorylation was examined by anti-pSmad2 or anti-pSmad3 immunoblotting of equivalent protein lysates (35 µg) prepared from plates transfected as indicated. Total levels of TLP, Smad2 and Smad3 expression are also shown (bottom panels). Occasionally, expression of exogenous TLP at medium-high concentrations appeared not to be dose dependent. This may reflect degradation of TLP, suggesting a strict control of TLP intracellular level above the physiologic threshold. (B) TLP has no effect on the kinetics of TGF-β-induced Smad2/3 phosphorylation. Hep3B cells were transiently transfected with a control vector (top panels) or with Xpr.TLP (middle panels), serum-starved for 18 h and treated with 5 ng/ml TGF-β1 for the indicated times. Phosphorylation of endogenous Smad2 and Smad3 was examined on equivalent protein lysates (70 µg) as described in (A). TLP expression is shown in the bottom panels.
TLP overexpression specifically blocks the formation of Smad3/4 complexes
The positioning of TLP effects downstream of Smad2 and Smad3 phosphorylation suggested to us that TLP might regulate the formation of Smad3/Smad4 and/or Smad2/Smad4 complexes. To test this hypothesis, we co-transfected COS-1 cells with Smad4 along with either Smad2 or Smad3, both in the absence and the presence of a constitutively active TβRΙ (Figure 8A). Immunoprecipita tion followed by immunoblotting showed that, while TLP resulted in a modest, if any, increase of Smad2/Smad4 complexes (Figure 8A, left panels), the association of Smad3 with Smad4 was strikingly inhibited by TLP expression, in both the basal and the activated state (Figure 8A, right panels). Thus the primary mechanism by which overexpressed TLP inhibits Smad3-dependent transcription is likely dependent on its ability to block TGF-β-induced Smad3/Smad4 complex formation. Experiments in HaCaT cells treated with TGF-β for 40 min again showed that levels of endogenous Smad2/Smad4 complexes were not changed by TLP overexpression, whereas the amount of endogenous Smad3/Smad4 complexes was markedly decreased in cells transfected with TLP in the absence of any effects on phosphorylation of either Smad2 or Smad3 (Figure 8B). No alteration in levels of either endogenous Smad3 or Smad4 was observed upon TLP transfection in HaCaT cells, suggesting that overexpressed TLP acts by inhibiting the formation or stability of Smad3/Smad4 heterodimers rather then by increasing the degradation of either binding partner of the complex. These experiments were repeated in mouse dermal fibroblasts with similar results (data not shown), indicating that TLP regulation of Smad3/Smad4 heterodimerization is part of a general mechanism regulating TGF-β signaling in both epithelial and mesenchymal cells.
Fig. 8. Overexpression of TLP blocks TGF-β-induced formation of Smad3/4 complexes while it does not alter Smad2/4 complex levels. (A) TLP effects on the formation of exogenous Smad2/4 and Smad3/4 complexes. COS-1 cells that co-expressed myc.Smad4 and Flag.Smad2 (left panel) or Flag.Smad3 (right panel) were transfected with HA.TβRI* along with a negative control vector (lanes 2 and 3) or with Xpr.TLP (lanes 4 and 5). Cells treated as in Figure 5A were immunoprecipitated with myc antibody and probed with anti-Flag. Leftmost lane: lysate of cells transfected with Flag.Smad2 (left panel) or Flag.Smad3 (right panel) cDNA alone was analyzed as negative control. Protein expression was confirmed by immunoblotting total cell lysates for the indicated tagged proteins (bottom panels). (B) TLP effects on the formation of endogenous Smad2/4 and Smad3/4 complexes. HaCaT cells, left untreated or stimulated with TGF-β (2.5 ng/ml) for 40 min, were treated with cross-linker for 20 min. The amount of endogenous Smad4 bound to Smad2 and Smad3 was determined by immunoprecipitation with anti-Smad2 and anti-Smad3 followed by immunoblotting with anti-Smad4, as indicated (top panels). Equal immunoprecipitations were confirmed by blotting anti-Smad2 or anti-Smad3 of the corresponding immunoprecipitates (second top panels). Steady state levels of endogenous Smad4 were analysed by anti-Smad4 blotting of total lysates (second bottom panels). Immunoblotting of total lysates with specific anti-pSmad2 and anti-pSmad3 antibodies shows endogenous activated Smad2 and Smad3 concentrations (bottom panels).
These results are consistent with the reporter assay data and indicate that while TLP upregulation has no effects on the levels of either Smad2/3/4 or R-Smad phosphorylation, it inhibits either the formation or the stability of TGF-β-dependent Smad3/Smad4 complexes, leading to a reduction in Smad3-dependent transcription. The observation that TLP overexpression does not increase Smad2 phosphorylation or Smad2/Smad4 complex formation, yet increases Smad2-dependent transcriptional responses (Figure 6), suggests that this effect is most likely due to release of the ARE promoter from Smad3 inhibition, similar to that seen previously in Smad3 null cells (Piek et al., 2001).
Reduction of endogenous TLP protein levels inhibits TGF-β-dependent transcription
To investigate further the role of TLP in regulating signaling flux through the Smad3 pathway, we transiently downregulated TLP expression in HaCaT cells by TLP-specific siRNAs, and determined the effects on TGF-β- and BMP-dependent transcription. While BMP-induced activation was not significantly affected by TLP downregulation, siRNA-mediated reduction of endogenous TLP levels strikingly inhibited Smad3-dependent transcription in HaCaT cells (Figure 9A). In these experiments, strong downregulation of TLP often decreased both the baseline and induced transcription, and negatively affected cell growth, suggesting that TLP may also play a role in regulation of other signaling pathways and/or also serve a more general function in the cell, as previously suggested (Caplan et al., 2001). These data indicate that TLP is a key regulator of TGF-β/activin signaling through Smad3, such that either downregulation of endogenous TLP levels or its possible mislocalization resulting from overexpression selectively compromise flux through the Smad3 pathway.
Fig. 9. Downregulation of endogenous TLP specifically blocks Smad3-dependent transcription. (A) siRNA-mediated TLP downregulation inhibits TGF-β-induced activation of Smad3/4 transcriptional response. HaCaT cells were transiently transfected with the indicated amounts of either control or TLP-specific siRNA followed by transfection with either SBE4 (left panel) or BRE (right panel). Luciferase activity was normalized for both transfection efficiency and protein concentration. (B) siRNA-dependent reduction of endogenous TLP protein levels. Aliquots of total lysates from HaCaT cells transfected as in (A) were tested in immunoblotting for endogenous TLP (top panel) and actin (bottom panel) expression.
Discussion
To our knowledge, TLP is the first molecule demonstrated to exhibit selective effects on Smad2- and Smad3-dependent signaling. The data demonstrating binding of TLP to TGF-β and activin type II receptors and selective inhibition of Smad3/Smad4 complex formation by deregulated TLP suggest that TLP is involved in localizing these receptors and Smad4 to specific intracellular compartments, where it regulates formation of Smad3/Smad4 but not Smad2/Smad4 complexes.
Possible role for TLP in endocytic trafficking
Several proteins known to affect TGF-β/activin signaling, including SARA (Tsukazaki et al., 1998), Dab2 (Hocevar et al., 2001), Hgs/Hrs (Miura et al., 2000) and Cav-1 (Razani et al., 2001) have been implicated in vesicle trafficking. Among these, SARA and Hgs/Hrs each contain a phosphatidylinositol 3-phosphate-binding FYVE domain, a characteristic of proteins localized to early endosomes, and have been proposed to function as a link between the TGF-β/Smad signaling pathway and endosomal membrane trafficking (Raiborg et al., 2001; Itoh et al., 2002 ; Panopoulou et al., 2002). In particular, SARA was suggested to affect Rab5-mediated endocytosis (Hu et al., 2002), while Hgs/Hrs was implicated in the recruitment of clathrin onto early endosomes (Raiborg et al., 2001) and in the sorting of ubiquitinated membrane proteins into clathrin-coated microdomains of early endosomes (Raiborg et al., 2002). Dab2, which associates with TGF-β receptors and Smad2/3, was recently shown to bind to the clathrin adaptor molecule AP-2 (Morris and Cooper, 2001) and to clathrin, and proposed to synchronize cargo selection and clathrin lattice polymerization events (Mishra et al., 2002). The presence of a clathrin heavy-chain repeat domain in TLP is particularly intriguing in terms of its potential involvement in endocytic transport. The clathrin heavy-chain repeat is a 145-residue motif, repeated seven times along the filamentous leg of clathrin, which polymerizes into polygonal lattices on the cytoplasmic surface of protein-coated membrane vesicles (Ybe et al., 1999). Clathrin-coated pits are critical sites of endocytic transport, with roles in activation and propagation of specific signaling cascades (McPherson, 2002). The clathrin repeat motif is also found in other non-clathrin proteins implicated in vacuolar maintenance and protein sorting in yeast (e.g. Vps 41/Vam2 and Vps39/Vam6), possibly mediating protein–protein interactions or representing a clathrin-binding domain (Nakamura et al., 1997). Deletion of the CLH domain in TLP/hVam6p resulted in loss of the ability of overexpressed protein to localize to lysosomes and to induce lysosomal clustering and fusion (Caplan et al., 2001), suggesting that it is important for its association with intracellular membrane vesicles.
Recent studies now link clathrin-mediated endocytosis with early events in TGF-β signaling. Whereas TβRI phosphorylation and association of SARA and Smad2 with the activated TβR complex occur at the plasma membrane, Smad2 and Smad3 phosphorylation and downstream signaling events, including formation of Smad2/4 and Smad3/4 complexes, only occur after clathrin-dependent endocytosis (Hayes et al., 2002; Penheiter et al., 2002). Although it has been shown that Smad2 and Smad3 activation occur in post-plasma membrane compartments, there is no evidence that Smad2 and Smad3 are phosphorylated in the same endocytic locale. Rather, data demonstrating that targeted deletion of either Smad2 or Smad3 in fibroblasts (Piek et al., 2001), or massive overexpression of either of these Smads (F.Tian and S.Yoo, unpublished data), has no effect on levels of TGF-β-dependent phosphorylation of the other Smads are consistent with the hypothesis that these two Smad proteins are phosphorylated by distinct receptor pools, possibly localized to different subcompartments of early endosomes (de Renzis et al., 2002). While SARA has been postulated to localize Smad2 to early endosomes (Seet and Hong, 2001; Panopoulou et al., 2002), at present little is known about the intracellular localization of Smad3 or the sites of Smad2/4 and Smad3/4 complex formation (Moustakas et al., 2001). However, controversialy, data addressing the stoichiometry of Smad complexes point to a different model of hetero-oligomerization of Smad2 and Smad3 with Smad4 (Inman and Hill, 2002; Moustakas and Heldin, 2002), which is consistent with a model in which these processes might be occurring in different accessory/scaffolding Smad signaling organizing centers.
TLP: a molecular switch regulating the balance of Smad3- versus Smad2-mediated TGF-β signaling
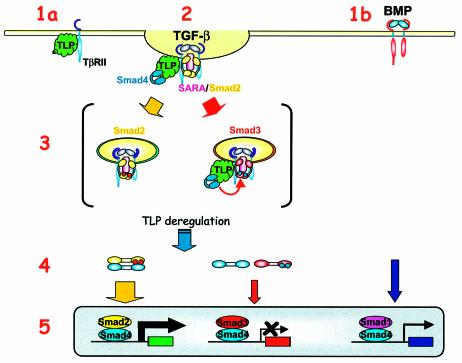
Based on this emerging view of the role of the endocytic compartment in Smad2/3 activation, we propose a model for TLP action based on both the presence of a CLH domain in TLP and our observations of (i) a cytoplasmic punctate staining pattern for overexpressed TLP, (ii) a constitutive association of TLP with the TβR complex, (iii) a ligand-dependent formation of TLP/Smad4 complexes and (iv) an inhibitory activity of deregulated TLP on Smad3/4 complex formation downstream of Smad2/3 phosphorylation with associated effects on signal propagation through the Smad3 pathway (Figure 10). In this model, TLP selectively associates with a subset of TβRII/activin RII in the absence of signaling (Figure 10, step 1a). Based on the assumption that Smad2- and Smad3-bound receptors might localize to distinct subdomains or subcompartments on endosomes, as has been shown for different Rab GTPases (de Renzis et al., 2002) and for SARA (Hu et al., 2002), we propose that TLP might direct activated receptors to Smad3-containing endocytic subdomains (Figure 10, step 2) and facilitate formation of Smad3/4 complexes (Figure 10, step 3). Deregulation of TLP interferes selectively with Smad3-dependent signaling (Figure 10, step 4) while possibly increasing certain Smad2-dependent transcriptional responses that are inhibited by Smad3 (Labbé et al., 1998; Piek et al., 2001) (Figure 10, step 5). To test this model it will be necessary to assess the subcellular localization of endogenous TLP and to determine how it is related to subcellular localization of endogenous Smad2, Smad3 and Smad4, and possibly also to FYVE domain proteins such as SARA and Hgs/Hrs, already implicated in linking vesicular trafficking and signal transduction.
Fig. 10. Proposed model for TLP opposite effects on Smad2- and Smad3-dependent transcriptional response to TGF-β. A model integrating defined early events in TGF-β signaling and a proposed mechanism of action for TLP is shown. Steps 1 and 2: TLP constitutive binding to TβRs, lack of association of TLP with BMPRs and association of TLP with Smad4 upon signal activation. Step 3: positioning of TLP downstream of Smad2 and Smad3 phosphorylation (described to occur on post-plasma membrane vesicles and here symbolized by red dots on Smad2 and blue dots on Smad3). Steps 4 and 5: TLP inhibition of Smad3/Smad4 complex formation and TLP effects on Smad-dependent transcription following deregulation of endogenous TLP levels. Although TLP is shown bound to Smad4 on the TβR complex throughout, our current observations do not address whether endocytic vesicle formation is required for TLP/Smad4 association. TLP, green; Smad4, blue; SARA, pink; Smad2, yellow; Smad3, red; Smad1, purple.
Overall, our observations support the hypothesis that TLP might function as an adaptor protein coupling TGF-β signaling to the endocytic compartment where it might act as a molecular switch to alter the balance of the Smad3- versus the Smad2-specific arm of TGF-β signaling. We are currently investigating alterations in cellular expression of TLP accompanying cellular differentiation or malignant transformation to assess whether it will contribute to changes in signaling flux through Smad2 and Smad3 and to shifts in transcriptional targets of TGF-β.
Materials and methods
Cell culture
COS-1, Hep3B and HaCaT cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS), 100 IU/ml penicillin and 100 µg/ml streptomycin (Life Technologies).
Cloning of TLP
A search of DNA databases identified a human homolog of TRAP-1 (KIAA0770) (Nagase et al., 2000). The complete open reading frame (ORF) of this cDNA, referred to here as TLP, was reconstructed by 5′-RACE from a HepG2 cDNA library using a gene-specific primer (5′-ctttctgtagtctgtggg-3′) that was designed on the published partial KIAA0770 cDNA sequence. Two nested PCRs with internal TLP primers (5′-gtgagatcatcctggc-3′ and 5-cacactaaagtccccctgc-3′) and adaptor primers were performed subsequently to amplify a 600 bp product that was cloned into pCR-II-TOPO (Invitrogen) and sequenced (NIH sequencing facility).
Sequence alignment and analysis
Sequence alignment of hTRAP-1 and hTLP was performed using the pairwise BLAST multiple sequence alignment program. The matrix was Blosum62, and ‘open gap’ and ‘extension gap’ penalties were 11 and 1, respectively. The ‘gap x_drop off’ value was set at 50 and the expect value at 10, with a word size of 3. Default parameters for the pblast algorithm (http://www.ncbi.nlm.nih.gov/blast/bl2seq/bl2.html) were used. Putative protein domains were analyzed using SMART (http://smart.embl-heidelberg.de/) as well as Prosite at http://www.isrec.isb-sib.ch/software/PFSCAN_form.html against Prosite pfamA/gribskov pattern library, with the sensitivity being set to include borderline matches.
Tissue distribution of TLP
Commercially available filters containing 2 µg/lane poly(A)+ RNA from multiple human tissues (Clontech) were prehybridized in ‘Church’ buffer [1% bovine serum albumen (BSA), 7% sodium dodecyl sulfate (SDS), 500 mM NaH2PO4 and 1 mM EDTA] at 70°C for 30 min and hybridized with a 32P-labeled partial TLP cDNA probe at 70°C in the same buffer o/n. Filters were washed at 70°C twice in 2× SSC/0.05% SDS and once in 0.1× SSC/0.1% SDS for 30 min each and subjected to autoradiography. The partial TLP probe was generated by PCR using TLP specific forward and backward primers (5′-cgggatccaccatgggggactttagtgtgcc-3′ and 5′-ctttctgtagtctgtggg-3′), leading to a 719 bp product at the 5′ end of the ORF.
Construction of epitope-tagged TLP constructs
The 5′-tagged full length TLP was generated using a PCR-based strategy. Human liver cDNA was generated from human liver RNA (Clontech), using Superscript reverse transcriptase (Life Technologies). Full-length TLP was amplified from this cDNA using ELONGASE proofreading enzyme (Life Technologies) and cloned into pcDNA4-HisMax-TOPO (Invitrogen). All constructs were fully sequence-verified.
Transient transfection, immunoprecipitation and western blotting
For co-immunoprecipitation experiments COS-1 cells were plated in 100 mm dishes at 1.8 × 106 cells/plate 24 h prior to transfection. Cells were transiently transfected with epitope-tagged receptors and TLP constructs using Lipofectamine (Life Technologies) according to the manufacturer’s instructions. Twenty-four hours after transfection the growing medium was replaced with DMEM–0.2%FBS. Forty-eight hours after transfection the cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed by the addition of 0.5 ml of lysis buffer (Wurthner et al., 2001) plus phosphatase and protease inhibitors. Where indicated, the cells were treated with 0.1mg/ml of dithiobis-succinimidyl propionate (DSP) (Pierce), a reducible chemical cross-linker, for 20 min at 37°C before lysis. Lysates were cleared by centrifugation, an aliquot of the lysate was removed for western blotting and the remainder was immunoprecipitated for 2 h with 0.4–1 µg of an epitope-specific antibody and 50 µl of protein G–Sepharose (Amersham Pharmacia Biotech). Immunoprecipitates were washed in lysis buffer four times, boiled in reducing sample-buffer (Novex) and separated by 10% SDS–polyacrylamide gels (Novex). Proteins were transferred onto Immobilon™ PVDF membranes (Millipore), detected using epitope-specific primary antibodies and horseradish-peroxidase-conjugated secondary antibodies (Amersham) and visualized by chemiluminescence (Pierce). Antibodies used were anti-HA mouse 12CA (hybridoma supernatant), anti-HA rabbit Y-11 (Santa Cruz Biotechnology), anti-Flag mouse M2 (Sigma), anti-Xpress mouse (Invitrogen), anti-GFP mouse (Zymed), anti-Smad2 and anti-Smad3 rabbit (Zymed), anti-Smad4 mouse [(Santa Cruz, for western blotting (WB)], anti-Smad4 rabbit [(Santa Cruz, for immunoprecipitation (IP)], anti-TβRII rabbit (L-21) (Santa Cruz, for IP), anti-TβRII goat (L-21) (Santa Cruz, for WB), anti-phospho Smad2 rabbit (Upstate Biotechnology) and anti-phospho Smad3 rabbit polyclonal (a gift from Dr M.Reiss). The rabbit antisera raised against TLP (749; 752) were used unpurified at 1:20 dilution in IP and 1:1000 dilution in WB. Specific blocking of the antibody with its antigen was performed by incubating the antibody with the blocking peptide (10ug/ml) for up 3 h at room temperature. Non-specific blocking was similarly carried out in the presence of an irrelevant blocking peptide.
Indirect immunofluorescence
COS-1 cells were plated at 3 × 105 cells onto 22 mm glass coverslips 24 h prior to transfection and transfected as described above. The cells were fixed in cold 3.5% paraformaldehyde for 5 min, permeabilized in cold methanol for 2 min and incubated for 5 min in 50 mM glycine. The transfected constructs were then detected by incubation with anti-HA rabbit polyclonal and anti-Xpress mouse monoclonal antibody for 2 h at room temperature. After washing in PBS, the coverslips were incubated for 1 h at room temperature with fluorescein isothiocyanate (FITC) conjugated goat anti-mouse IgG and rhodamine (TRITC) conjugated goat anti-rabbit IgG secondary antibodies (Molecular Probes). The coverslips were then mounted in medium containing 4,6-diamino-2-phenylindole (DAPI) (Vector Laboratories) and examined using a Leica TCS.NT laser scanning confocal microscope. Appropriate emission filters settings and controls were included to exclude bleed-through effects.
Luciferase reporter assays
Hep3B cells were plated at 2.5 × 105cells/well in six-well plates 24 h prior to transfection. The cells were transfected with the indicated amounts of Xpr.TLP, reporter plasmid (ARE-Luc/FAST-1, BRE-Luc, SBE4-Luc, Smad7-Luc), pSVβ-Gal to normalize transfection efficiency and pcDNA3 to normalize the amount of transfected DNA. After 24 h the transfection medium was replaced with DMEM/0.2%FBS and the cells were left untreated or stimulated with 5 ng/ml TGF-β1 for 18 h. Transcriptional reporter assays with the human Smad7 promoter were performed as previously described (von Gersdorff et al., 2000). The luciferase activity was measured using the luciferase assay system (PharMingen) and normalized for transfection efficiency to β-Gal expression. All assays were performed in triplicate.
RNA oligonucleotides and siRNA transfection
siRNA oligonucleotides with two thymidine residues (dTdT) at the 3′ end of the sequence were designed to TLP (sense DNA target sequence, 5′-AACTGCCTGATCCAGCTATAC-3′) which extend between amino acids 609 and 615 along with the corresponding antisense oligonucleotides, as previously described (Elbashir et al., 2001) (Xeragon). The siRNA duplex was resuspended in sterile buffer (100 mM potassium acetate, 30 mM HEPES–KOH, 2 mM magnesium acetate pH 7.4) at 40 µM concentration, heated at 90°C for 1 min and then incubated at 37°C for 60 min to disrupt higher aggregates. For luciferase assays, 2.5 × 105 cells/well were plated in a six-well plate 24 h prior to transfection. The cells were transiently transfected with the indicated amounts (0.05–1 µg/well) of either TLP siRNA or non-silencing control siRNA (80–11310; Xeragon) using Lipofectamine (Invitrogen) in serum-free medium for 7 h, and then switched to 10% FBS-containing medium. To determine the effect of TLP siRNA on reporter constructs, the cells were transfected with either SBE4 or BRE and pSVβ-gal 18 h after siRNA transfection. Eighty hours after siRNA transfection, the cells were starved and treated with either TGF-β1 (5 ng/ml) or BMP-2 (100 ng/ml) for 18 h. Four days after siRNA transfection, the luciferase activity was measured and normalized to β-Gal expression and to protein content.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Dr Jeff Wrana and Dr Liliana Attisano for TβRs constructs, Dr Peter ten Djike for the BRE-Luc, Dr Seong-Jin Kim for the tumor cell line RNA, Dr Erwin Bottinger for the pS7-1-Luc reporter and Dr Takahiro Nagase (Kazusa Institute) for the KIAA0770 clone. This study was supported in part by a stipend from the Deutsche Forschungsgemeinschaft to J.U.W.
References
- Altschul S.F., Madden,T.L., Schaffer,A.A., Zhang,J., Zhang,Z., Miller,W. and Lipman,D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan S., Hartnell,L.M., Aguilar,R.C., Naslavsky,N. and Bonifacino,J.S. (2001) Human Vam6p promotes lysosome clustering and fusion in vivo. J. Cell Biol., 154, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charng M.J., Zhang,D., Kinnunen,P. and Schneider,M.D. (1998) A novel protein distinguishes between quiescent and activated forms of the type I transforming growth factor beta receptor. J. Biol. Chem., 273, 9365–9368. [DOI] [PubMed] [Google Scholar]
- Chen R.H., Miettinen,P.J., Maruoka,E.M., Choy,L. and Derynck,R. (1995) A WD-domain protein that is associated with and phos phorylated by the type II TGF-beta receptor. Nature, 377, 548–552. [DOI] [PubMed] [Google Scholar]
- Datta P.K. and Moses,H.L. (2000) STRAP and Smad7 synergize in the inhibition of Transforming growth factor β signaling. Mol. Cell. Biol., 20, 3157–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Caestecker M.P., Piek,E. and Roberts,A.B. (2000) Role of transforming growth factor-beta signaling in cancer. J. Natl Cancer Inst., 92, 1388–1402. [DOI] [PubMed] [Google Scholar]
- de Renzis S., Sonnichsen,B. and Zerial,M. (2002) Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat. Cell Biol., 4, 124–133. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo G.M., Le Roy,C., Goodfellow,A.F. and Wrana,J.L. (2003) Distinct endocytic pathways regulate TGF-b receptor signaling and turnover. Nat. Cell Biol., 5, 410–421. [DOI] [PubMed] [Google Scholar]
- Doré J.J.E. Jr, Edens,M., Garamszegi,N. and Leof,E.B. (1998) Heteromeric and homomeric transforming growth factor-β receptors show distinct signaling and endocytic responses in epithelial cells. J. Biol. Chem., 273, 31770–31777. [DOI] [PubMed] [Google Scholar]
- Elbashir S.M., Harboth,J., Lendeckel,W., Yalcin,A., Weber,K. and Tuschl,T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494–498. [DOI] [PubMed] [Google Scholar]
- Griswold-Prenner I., Kamibayashi,C., Maruoka,E.M., Mumby,M.C. and Derynck,R. (1998) Physical and functional interactions between type I transforming growth factor beta receptors and Balpha, a WD-40 repeat subunit of phosphatase 2A. Mol. Cell. Biol., 18, 6595–6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S., Chawla,A. and Corvera,S. (2002) TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J. Cell Biol., 158, 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar B.A., Smine,A., Xu,X-X. and Howe,P.H. (2001) The adaptor molecule Disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO J., 20, 2789–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Chuang,J.Z., Xu,K., McGraw,T.G. and Sung,C.H. (2002) SARA, a FYVE domain protein, affects Rab5-mediated endocytosis. J. Cell Sci., 115, 4755–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman G.J. and Hill,C. (2002) Stoichiometry of active Smad-transcription factor complexes on DNA. J. Biol. Chem., 277, 51008–51016. [DOI] [PubMed] [Google Scholar]
- Itoh F., Divecha,N., Brocks,L., Oomen,L., Janssen,H., Calafat,J., Itoh,S. and Dijke,Pt.P. (2002) The FYVE domain in Smad anchor for receptor activation (SARA) is sufficient for localization of SARA in early endosomes and regulates TGF-beta/Smad signaling. Genes Cells, 7, 321–331. [DOI] [PubMed] [Google Scholar]
- Korchynskyi O. and ten Dijke,P. (2002) Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem., 277, 4883–4891. [DOI] [PubMed] [Google Scholar]
- Labbé E., Silvestri,C., Hoodless,P.A., Wrana,J.L. and Attisano,L. (1998). Smad2 and Smad3 positively and negatively regulate TGF-dependent transcription through the forkhead DNA binding protein FAST2. Mol. Cell, 2, 109–120. [DOI] [PubMed] [Google Scholar]
- McPherson PS (2002) The endocytic machinery at an interface with the actin cytoskeleton: a dynamic, hip intersection. Trends Cell Biol., 12, 312–315. [DOI] [PubMed] [Google Scholar]
- Macias-Silva M., Abdollah,S., Hoodless,P.A., Pirone,R., Attisano,L. and Wrana,J.L. (1996) MADR2 is a substrate of the TGFbeta receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell, 87, 1215–1224. [DOI] [PubMed] [Google Scholar]
- Madaule P., Furuyashiki,T., Reid,T., Ishizaki,T., Watanabe,G., Morii,N. and Narumiya,S. (1995) A novel partner for the GTP-bound forms of rho and rac. FEBS Lett., 377, 243–248. [DOI] [PubMed] [Google Scholar]
- Massague J. and Chen,Y.G. (2000) Controlling TGF-beta signaling. Genes Dev., 14, 627–644. [PubMed] [Google Scholar]
- Massague J., Blain,S.W. and Lo,R.S. (2000) TGFbeta signaling in growth control, cancer and heritable disorders. Cell, 103, 295–309. [DOI] [PubMed] [Google Scholar]
- Mishra S.K., Keyel,P.A., Hawryluk,M.J., Agostinelli,N.R., Watkins,S.C. and Traub,L.M. (2002) Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J., 21, 4915–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S. et al. (2000) Hgs (Hrs), a FYVE domain protein, is involved in Smad signaling through cooperation with SARA. Mol. Cell. Biol., 20, 9346–9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K., ten Dijke,P. and Heldin,C.H. (2000) TGF-beta signaling by Smad proteins. Adv. Immunol., 75, 115–157. [DOI] [PubMed] [Google Scholar]
- Morris S.M. and Cooper,J.A. (2001) Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic, 2, 111–123. [DOI] [PubMed] [Google Scholar]
- Moustakas A. and Heldin,C.H. (2002) From mono- to oligo-Smads: the heart of the matter in TGF-signal transduction. Genes Dev., 16, 1867–1871. [DOI] [PubMed] [Google Scholar]
- Moustakas A., Souchelnytskyi,S. and Heldin,C.H. (2001) Smad regulation in TGF-beta signal transduction. J. Cell Sci., 114, 4359–4369. [DOI] [PubMed] [Google Scholar]
- Nagarajan R.P., Liu,J. and Chen,Y. (1999) Smad3 inhibits transforming growth factor-beta and activin signaling by competing with Smad4 for FAST-2 binding. J. Biol. Chem., 274, 31229–31235. [DOI] [PubMed] [Google Scholar]
- Nagase T., Kikuno,R., Ishikawa,K., Hirosawa,M. and Ohara,O. (2000) Prediction of the coding sequences of unidentified human genes. XVII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res., 7, 143–150. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Hirata,A., Ohsumi,Y. and Wada,Y. (1997) Vam2/Vps41p and Vam6/Vps39p are components of a protein complex on the vacuolar membranes and involved in the vacuolar assembly in the yeast Saccharomyces cerevisiae. J. Biol. Chem., 272, 11344–11349. [DOI] [PubMed] [Google Scholar]
- Panopoulou E., Gillooly,D.J., Wrana,J.L., Zerial,M., Stenmark,H., Murphy,C. and Fotsis,T. (2002) Early endosomal regulation of Smad-dependent signaling in endothelial cells. J. Biol. Chem., 277, 18046–18052. [DOI] [PubMed] [Google Scholar]
- Penheiter S.G., Mitchell,H., Garamszegi,N., Edens,M., Dore,J.J.,Jr and Leof,E.B. (2002) Internalization-dependent and -independent requirements for transforming growth factor beta receptor signaling via the Smad pathway. Mol. Cell. Biol., 22, 4750–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R., Schiemann,W.P., Brooks,M.W., Lodish,H.F. and Weinberg,R.A. (2001) TGF-beta-induced apoptosis is mediated by the adaptor protein Daxx that facilitates JNK activation. Nat. Cell Biol., 8, 708–714. [DOI] [PubMed] [Google Scholar]
- Piek E. et al. (2001) Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J. Biol. Chem., 276, 19945–19953. [DOI] [PubMed] [Google Scholar]
- Raiborg C., Bache,K.G., Mehlum,A., Stang,E. and Stenmark,H. (2001) Hrs recruits clathrin to early endosomes. EMBO J., 20, 5008–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg C., Bache,K.G., Gillooly,D.J., Madshus,I.H., Stang,E. and Stenmark,H. (2002) Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol., 4, 394–398. [DOI] [PubMed] [Google Scholar]
- Razani B., Zhang,X.L., Bitzer,M., von Gersdorff,G., Bottinger,E.P. and Lisanti,M.P. (2001) Caveolin-1 regulates transforming growth factor (TGF)-b/SMAD signaling through an interaction with the TGF-β type I receptor. J. Biol. Chem., 276, 6727–6738. [DOI] [PubMed] [Google Scholar]
- Roberts A.B. and Sporn,M.B. (1990) The transforming growth factors-b. In Sporn,M.B. and Roberts,A.B. (eds), Handbook of Experimental Pharmacology. Peptide Growth Factors and Their Receptors. Springer-Verlag, Berlin, pp. 419–472. [Google Scholar]
- Schultz J., Copley,R.R., Doerks,T., Ponting,C.P. and Bork,P. (2000) SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res., 28, 231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet L.F. and Hong,W. (2001) Endofin, an endosomal FYVE domain protein. J. Biol. Chem., 276, 42445–42454. [DOI] [PubMed] [Google Scholar]
- Tsukazaki T., Chiang,T.A., Davison,A.F., Attisano,L. and Wrana,J.L. (1998) SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell, 95, 779–791. [DOI] [PubMed] [Google Scholar]
- von Gersdorff G., Susztak,K., Rezvani,F., Bitzer,M., Liang,D. And Bottinger,E.P. (2000) Smad3 and Smad4 mediate transcriptional activation of the human Smad7 promoter by transforming growth factor B. J. Biol. Chem., 275, 11320–11326. [DOI] [PubMed] [Google Scholar]
- Wang T., Li,B.Y., Danielson,P.D., Shah,P.C., Rockwell,S., Lechleider,R.J., Martin,J., Manganaro,T. and Donahoe,P.K. (1996) The immunophilin FKBP12 functions as a common inhibitor of the TGF beta family type I receptors. Cell, 86, 435–444. [DOI] [PubMed] [Google Scholar]
- Weinstein M., Yang,X. and Deng,C (2000) Functions of mammalian Smad genes as revealed by targeted gene disruption in mice. Cytokine Growth Factor Rev., 11, 49–58. [DOI] [PubMed] [Google Scholar]
- Weis-Garcia F. and Massague,J. (1996) Complementation between kinase-defective and activation-defective TGF-beta receptors reveals a novel form of receptor cooperativity essential for signaling. EMBO J., 15, 276–289. [PMC free article] [PubMed] [Google Scholar]
- Wieser R., Wrana,J.L. and Massague,J. (1995) GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J., 14, 2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurthner J.U., Frank,D.B., Felici,A., Green,H.M., Cao,Z., Schneider,M.D., McNally,J.G., Lechleider,R.J. and Roberts,A.B. (2001) Transforming growth factor-beta receptor-associated protein 1 is a Smad4 chaperone. J. Biol.Chem., 276, 19495–19502. [DOI] [PubMed] [Google Scholar]
- Yagi K., Goto,D., Hamamoto,T., Takenoshita,S., Kato,M. and Miyazono,K. (1999) Alternatively spliced variant of Smad2 lacking exon 3. Comparison with wild-type Smad2 and Smad3. J. Biol. Chem., 274, 703–709. [DOI] [PubMed] [Google Scholar]
- Yao D., Ehrlich,M., Henis,Y.I. and Leof,E.B. (2002) Transforming growth factor-β receptors interact with AP2 by direct binding to β2 subunit. Mol. Biol. Cell, 13, 4001–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybe J.A., Brodsky,F.M., Hofmann,K., Lin,K., Liu,S.H., Chen,L., Earnest,T.N., Fletterick,R.J. and Hwang,P.K. (1999) Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature, 399, 371–375. [DOI] [PubMed] [Google Scholar]
- Zawel L., Dai,J.L., Buckhaults,P., Zhou,S., Kinzler,K.W., Vogelstein,B and Kern,S.E. (1998) Human Smad3 and Smad4 are sequence-specific transcription activators. Mol. Cell, 1, 611–617. [DOI] [PubMed] [Google Scholar]