Abstract
Contraction of vascular smooth muscle cells (VSMCs) depends on the rise of cytosolic [Ca2+] owing to either Ca2+ influx through voltage-gated Ca2+ channels of the plasmalemma or receptor-mediated Ca2+ release from the sarcoplasmic reticulum (SR). We show that voltage-gated Ca2+ channels in arterial myocytes mediate fast Ca2+ release from the SR and contraction without the need of Ca2+ influx. After sensing membrane depolarization, Ca2+ channels activate G proteins and the phospholipase C–inositol 1,4,5-trisphosphate (InsP3) pathway. Ca2+ released through InsP3-dependent channels of the SR activates ryanodine receptors to amplify the cytosolic Ca2+ signal. These observations demonstrate a new mechanism of signaling SR Ca2+-release channels and reveal an unexpected function of voltage-gated Ca2+ channels in arterial myocytes. Our findings may have therapeutic implications as the calcium-channel-induced Ca2+ release from the SR can be suppressed by Ca2+- channel antagonists.
Keywords: arterial myocytes/Ca2+ channels/calcium-channel-induced Ca2+ release/inositol trisphosphate/voltage sensor
Introduction
Vascular smooth muscle cells (VSMCs) control blood flow and pressure. Contraction of these cells depends on the rise of cytosolic [Ca2+], which can occur owing to either Ca2+ entry through membrane channels or to receptor-mediated Ca2+ release from the sarcoplasmic reticulum (SR) (Somlyo and Somlyo, 1994; Bolton et al., 1999). It has previously been reported that exposure to high extracellular K+ can induce elevation of cytosolic [Ca2+] in VSMCs without the need of significant transmembrane Ca2+ influx (Kobayashi et al., 1986). In addition, it is known that agonist-induced Ca2+ release from internal stores can be altered by modifications of the membrane potential in several cell types (Itoh et al., 1992; Ganitkevich and Isenberg, 1993; Kukuljan et al., 1994; Jaimovich et al., 2000; Mason and Mahaut-Smith, 2001). However, it is not known whether membrane electrical events in vascular smooth muscle can per se activate the Ca2+ release channels of the SR as well as the underlying mechanisms. Herein we show that in myocytes dispersed from basilar brain arteries, membrane depolarization can evoke Ca2+ release from stores that are sensitive to inositol 1,4,5-trisphosphate (InsP3) and ryanodine (Ry) (Ehrlich and Watras, 1988; Blatter and Weir, 1992; Berridge, 1993; Pozzan et al., 1994) in a graded manner and can produce cell contraction in the absence of agonists or extracellular Ca2+ influx. Coupling of myocyte depolarization to Ca2+ release from the SR depends on voltage-gated Ca2+ channels that, after sensing the change of membrane potential, activate G proteins and subsequently the phospholipase C–InsP3 pathway. Ca2+ released through InsP3-dependent channels activates Ry receptors to amplify the cytosolic Ca2+ signal. These observations demonstrate a new mechanism of signaling SR Ca2+-release channels and reveal an unexpected functional feature of plasmalemmal voltage-gated Ca2+ channels in VSMCs. In addition to their well-known role as Ca2+-selective membrane pores, they act as voltage sensors capable of triggering fast G-protein-dependent Ca2+ release from the SR and myocyte contraction.
Results
Calcium-channel-induced Ca2+ release and myocyte contraction in the absence of extracellular Ca2+ influx
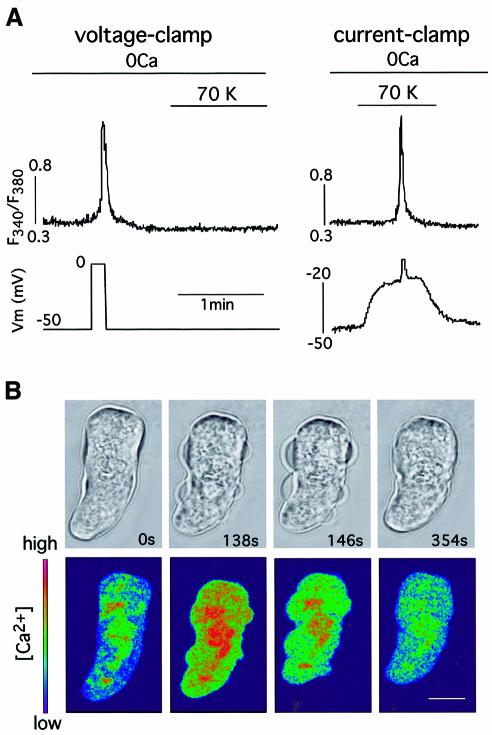
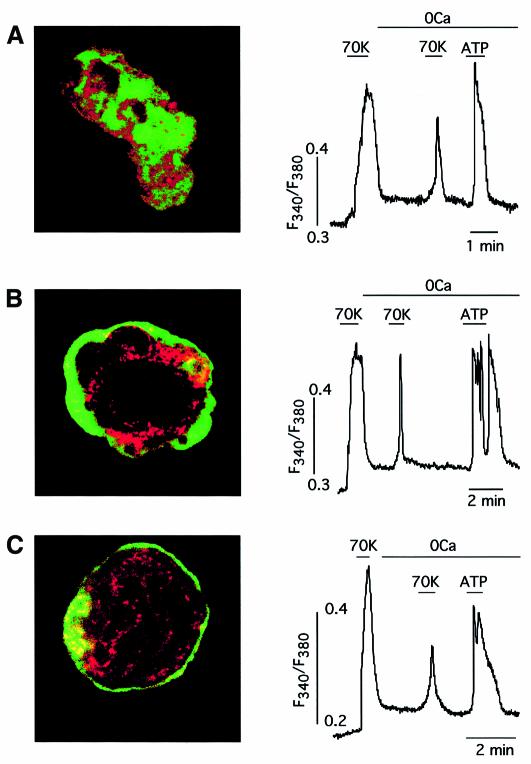
In voltage-clamped basilar arterial myocytes, loaded with Fura-2 and bathed in solutions without external Ca2+, membrane depolarization induced a fast transient Ca2+ signal due to release of the cation from internal stores (Figure 1A, left). The signal normally had a slow foot lasting a few seconds preceding a sharp rise of [Ca2+]. This depolarization-induced Ca2+ release was also triggered in current-clamped cells (Figure 1A, right) or in intact undialyzed, myocytes (see below) by depolarization with 70 mM extracellular K+. However, high external K+ failed to elicit depolarization-induced Ca2+ release in voltage-clamped cells with the membrane potential held at –50 mV (Figure 1A), suggesting that it primarily requires a change in the cell’s membrane potential. Depolarization-induced Ca2+ release has been observed in 81% of the basilar myocytes studied (n = 232) bathed in either Ca2+-free solutions (0 mM Ca2+ plus 2 mM EGTA) or in external solutions with cadmium (0.4 mM) and nickel (3 mM) added to block transmembrane Ca2+ influx. Depolarization-induced Ca2+ release was also present in Ca2+- and Na+-free solutions (Na+ substituted for choline; n = 13 cells tested) but totally abolished by previous exposure to thapsigargin (300 nM; n = 7 cells tested), a blocker of SR Ca2+-ATPase (Thastrup et al., 1990), thus indicating that the SR was the source of the Ca2+ released by membrane depolarization. In myocytes bathed in an external solution without Ca2+ and with 2 mM EGTA added, depolarization was able to produce contraction, as demonstrated by confocal microscopy studies in which we simultaneously monitored changes in cell shape and cytosolic [Ca2+] (Figure 1B). Depolarization-induced Ca2+ release was observed as a graded phenomenon whose magnitude increased in parallel with that of membrane depolarization. In voltage-clamped myocytes depolarizing pulses of increasing amplitude elicited progressively larger Ca2+-release signals that triggered outward currents due to activation of Ca2+-dependent maxi-K+ channels known to be present in arterial myocyte membranes (Figure 2A) (Nelson et al., 1995; Smani et al., 2001). The amplitude of the Ca2+-release signal (measured as change of fluorescence ratio) as a function of the membrane potential is plotted in Figure 2B using data from several voltage-clamped cells held at –90 mV. In these conditions, the threshold for the appearance of depolarization-induced Ca2+ release was ∼–70 mV and the midpoint of the fitted Boltzmann function was at ∼–15 mV. The similarity of the curve in Figure 2B to the charge movement versus membrane potential relation for the L-type Ca2+ channels (Hadley and Lederer, 1991) suggested that these channels, highly expressed in vascular smooth muscle, might be the voltage sensors coupling the changes of myocyte membrane potential to Ca2+ release from the SR. In fair agreement with this hypothesis, depolarization-induced Ca2+ release elicited by fast depolarizing pulses was markedly reduced (by 82.2 ± 8.1%, n = 6) when the same level of depolarization was obtained by slowly changing the membrane potential (Figure 2C). This observation suggested that, similar to what occurs in voltage-gated Ca2+ channels, the slow ramp-like depolarization resulted in inactivation of the voltage sensor.
Fig. 1. Membrane depolarization induces Ca2+ release and contraction in myocytes bathed in Ca2+-free solutions. (A) Left: membrane-depolarization-induced sharp Ca2+-release signal in a voltage-clamped basilar myocyte bathed in Ca2+-free external solution (0Ca plus 2 mM EGTA added). Addition of 70 mM external K+ (70K) failed to produce membrane depolarization and Ca2+ release. Right: Ca2+ release induced by depolarization with external K+ (70K) in the same myocyte subjected to current clamp. (B) Time course of contraction (top) and cytosolic [Ca2+] (bottom) in a myocyte loaded with Fluo3-AM and bathed in a Ca2+-free external solution for 5 min. At time zero, the cell was depolarized by application of 70 mM external K+ (Ca2+-free) for 140 s. The color scale (arbitrary units) is shown in the lower left corner. Calibration bar, 10 µm. Average reduction of cell length measured in the larger axis was 16 ± 6% (n = 4 cells).
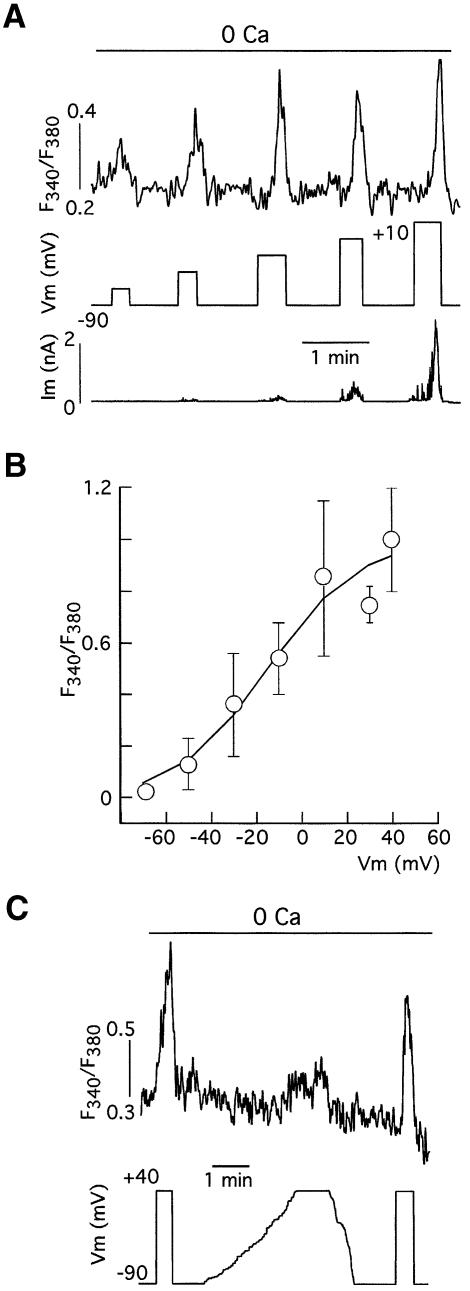
Fig. 2. Graded voltage dependence of Ca2+ release induced by membrane depolarization. (A) Progressive increase in the Ca2+ release signal (expressed as change in the fluorescence ratio) in a voltage-clamped myocyte in parallel with the amplitude of depolarizing steps. (B) Average changes of fluorescence ratio versus membrane potential relationship in basilar myocytes. Data points represent the mean ± SE from n = 13 cells. The values were normalized to the amplitude at +40 mV. The curve is a least-squares fit to a Boltzmann function. V1/2 = –15 mV and k = 20 mV. (C) Depolarization-induced Ca2+ release is abolished when the membrane depolarization is changed slowly. Representative example of qualitatively similar experiments performed on six cells.
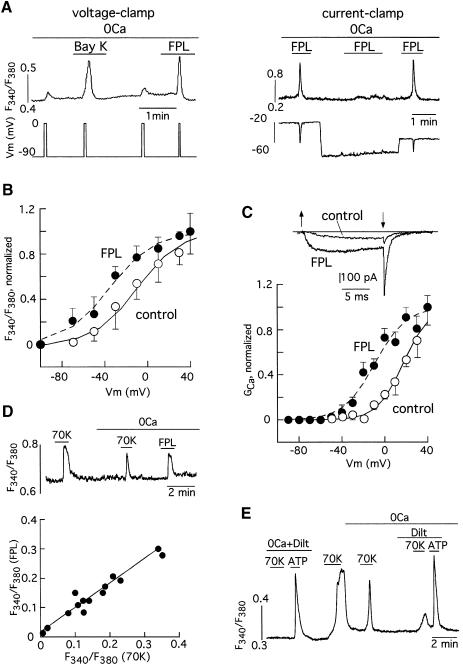
The resemblance between the voltage sensor that mediates depolarization-induced Ca2+ release and the voltage-dependent Ca2+ channels was further evidenced by pharmacological experiments. Depolarization-induced Ca2+ release was potentiated by the selective Ca2+-channel agonists Bay K-8644 and, with higher potency, FPL-64176, a benzoylpyrrole derivative that alters Ca2+-channel gating by binding to a site other than the dihydropyridine receptor (Figure 3A, left) (Bean et al., 1986; Zheng et al., 1991). Figure 3A (right) illustrates that Ca2+ release induced by FPL was prevented by membrane hyperpolarization in a current-clamped myocyte. FPL-induced Ca2+ release was abolished in cells maintained at membrane voltages more negative than –70 mV (n = 6 cells tested), thus indicating that the effect of the drug was to reduce the membrane potential threshold required for depolarization-induced Ca2+ release. In fact, addition of FPL produced a displacement of ∼20 mV to negative voltages of the curve relating Ca2+ release amplitude and membrane potential (Figure 3B) and a qualitatively similar shift in the Ca2+ conductance versus voltage relation (Figure 3C). The differences observed in the half activation voltage for depolarization-induced Ca2+ release (open symbols in Figure 3B) and calcium currents (open symbols in Figure 3C) can be explained, at least in part, by the different concentration of external divalent cations used for these experiments (respectively, 0 mM Ca2+ versus 10 mM Ba2+). In addition, it is expected that the intramembranous charge movement triggering depolarization-induced Ca2+ release could occur at voltages more negative than those required for the actual opening of the calcium channels. In addition to the similarity of the effects of FPL on depolarization-induced Ca2+ release and Ca2+-channel gating, the magnitude of the Ca2+-release signals evoked by high K+ or FPL varied in parallel in different cells (Figure 3D), thus suggesting that both stimuli (membrane depolarization and pharmacological activation of Ca2+ channels) act through a common mechanism. Finally, depolarization-induced Ca2+ release was almost completely abolished by diltiazem (10–50 µM; 87.8 ± 1.7% inhibition, n = 13 cells tested), a widely used L-type Ca2+-channel antagonist in vascular smooth muscle (Kuga et al., 1990; Kraus et al., 1998), although this drug did not alter ATP-induced Ca2+ release (see Figure 3E). Other Ca2+-channel antagonists as well as diltiazem reduced depolarization-induced Ca2+ release with variable potency. D600 (10–50 µM) was as effective as diltiazem and blocked depolarization-induced Ca2+ release by 96.5 ± 1.5% (n = 10). Interestingly, nifedipine (5 µM) significantly reduced depolarization-induced Ca2+ release amplitude in only seven of 17 cells tested and had a relatively low potency to block the macroscopic calcium current (52.7 ± 7% reduction of peak current amplitude at +20 mV, n = 15). However, nifedipine, similarly to diltiazem and D600, completely abolished FPL-induced Ca2+ release in all the cells tested (n = 4). These findings demonstrate that in the complete absence of transmembrane Ca2+ influx, activation of L-type Ca2+ channels of basilar VSMCs by either membrane depolarization or pharmacological agonists triggers a fast Ca2+-release signal from intracellular stores of sufficient amplitude to produce contraction of the cells. Therefore we will refer to this phenomenon as calcium-channel-induced calcium release (CCICR) in the rest of this article.
Fig. 3. Calcium-channel-induced Ca2+ release and effects of Ca2+-channel agonists and antagonists. (A) Left: Ca2+ release evoked by the application of membrane-depolarizing pulses in a voltage-clamped myocyte bathed in the Ca2+-free solution and potentiation of this effect by addition of the Ca2+-channel agonists Bay K (200 nM) and FPL ( 200 nM). Right: Ca2+ release evoked by the application of FPL (1 µM) in a current-clamped myocyte is prevented by membrane hyperpolarization. Fast hyperpolarizations occurring in parallel with the Ca2+ transients are due to activation of Ca2+-activated K+ channels. In the first current injection (75 pA) the membrane potential was changed from –20 to –90 mV, thus yielding a value of input resistance of 0.93 GΩ. (B) Average Ca2+ release (indicated as fluorescence ratio) versus membrane potential relationship in basilar myocytes before (open symbols; see Figure 2B) and after (filled symbols; mean ± SE from n = 11 cells) exposure to FPL (30 nM). Curves are least-squares fits to Boltzmann functions (see Figure 2). For curve in FPL, V1/2 = –35.2 mV and k = 20.9 mV. Note that FPL shifted V1/2 by –20.2 mV. Fluorescence values obtained at –30 and –10 mV in the two sets of data were statistically different (p = 0.03 and p = 0.018 respectively). (C) Normalized calcium conductance versus membrane potential relationship obtained in the barium solution before (open symbols) and after (filled symbols) addition of 200 nM FPL (n = 5 cells). Curves are least-squares fits to Boltzmann functions. For curve control, V1/2 = 17.2 mV and k = 12.1 mV, and for curve FPL V1/2 = –9.1 mV and k = 14.6 mV. Note that FPL shifted V1/2 by –26.3 mV. The inset shows superimposed examples of calcium currents generated by depolarization (between the arrows) to +20 mV from –80 mV in the two experimental conditions. (D) Top: Ca2+ release induced by FPL (1 µM) and by depolarization with 70 mM K+ in a representative cell. Bottom: relationship between the amplitude of the Ca2+ signals evoked by FPL (ordinate) and by 70 mM K+ (abscissa) in 14 cells. Linear regression fit (r = 0.95). (E) Blockade of Ca2+-release induced by membrane depolarization in the presence of diltiazem (10 µM). Note that Ca2+ release induced by 100 µM ATP (0.26 ± 0.09 increase in fluorescence ratio, n = 12) is not significantly affected by the presence of 10–50 µM diltiazem (0.22 ± 0.025 increase in fluorescence ratio, n = 12, p > 0.05).
Calcium channel-induced Ca2+ release requires G-protein-mediated activation of the phospholipase C–inositol trisphosphate pathway
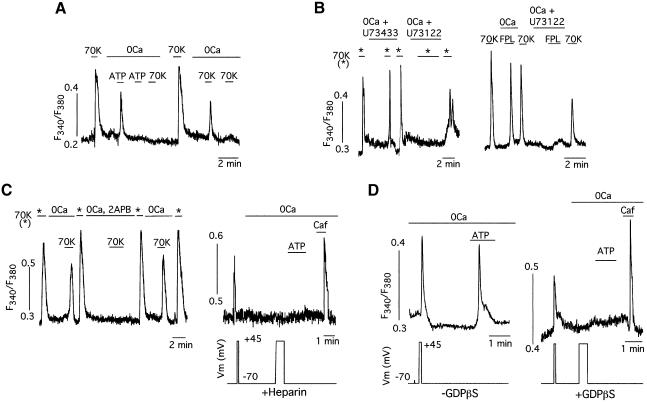
To identify the transduction pathway and the internal stores involved in CCICR we performed the series of experiments summarized in Figure 4. Pretreatment of the cells with ATP, which is known to elicit InsP3-dependent Ca2+ release in VSMCs of the basilar artery (Sima et al., 1997), depleted the intracellular stores and prevented any further CCICR upon exposure to high K+ (Figure 4A). These observations suggested that both ATP-induced Ca2+ release and CCICR operate through the same Ca2+ stores. Ca2+ release induced by either high K+ or FPL was fully prevented by incubation of the cells with U73122, an inhibitor of phospholipase C (Smith et al., 1990), whereas U73433, a related drug that does not affect the enzyme, was ineffective (Figure 4B; n = 9 cells tested). CCICR was also reversibly abolished by 2APB, a blocker of the InsP3-sensitive Ca2+-release channels (Figure 4C left; n = 10 cells tested) (Maruyama et al., 1997). In addition, CCICR or ATP-evoked Ca2+ release were totally abolished in patch–clamped cells dialyzed with heparin, an inhibitor of the InsP3 receptors (Ehrlich and Watras, 1988) (Figure 4C, right; n = 7 cells tested). Together, these findings indicated that CCICR resulted from phospho lipase C activation and InsP3 production, which in turn elicited Ca2+ release from SR. Activation of phospholipase C by membrane depolarization involved G-protein function, as cell dialysis with 1 mM GDPβS prevented depolarization-induced or ATP-dependent Ca2+ release in all cells studied (n = 25). Both voltage-dependent extracellular Ca2+ influx and Ry receptor stimulation by caffeine were unaltered by dialysis with GDPβS (Figure 4D).
Fig. 4. Calcium-channel-induced Ca2+ release from sarcoplasmic reticulum is mediated by G-protein-dependent InsP3 production. (A) Abolition of CCICR observed with 70 mM K+ after repeated exposure of a myocyte to ATP (100 µM) in Ca2+-free external solutions. (B) Suppression of CCICR evoked with either 70 mM K+ (left) or FPL (1 µM, right) by application of an inhibitor of phospholipase C (U73122, 1 µM). Note that the analog U73433 (1 µM) has no effect. (C) Left: abolition of CCICR evoked with 70 mM K+ by application of a blocker of InsP3 receptors (2APB, 50 µM). Right: suppression of depolarization- and ATP (100 µM)-induced Ca2+ release in a representative patch–clamped myocyte loaded with heparin (200 µg/ml) to inhibit InsP3 receptor activation. Note that the response to caffeine (Caf, 5 mM), a Ry receptor agonist, was unaltered. (D) Left: CCICR elicited in voltage-clamped cells dialyzed with the normal internal solution (–GDPβS). A similar Ca2+ release signal is induced by membrane depolarization or the application of ATP (100 µM). Right: abolition of depolarization- or ATP-induced Ca2+ release in cells dialyzed with GDPβS (1 mM, added to the patch pipette solution). Note that before switching to the Ca2+-free solution (0Ca) a brief depolarizing pulse produced a Ca2+ signal due to influx through membrane channels and that G protein blockade did not prevent Ca2+ release induced by caffeine (Caf, 5 mM). GDPβS abolished CCICR in 100% of the cells tested (n = 25). In the same series of experiments, ∼90% of the patch–clamped cells dialyzed without GDPβS (n = 33) responded with Ca2+ release to membrane depolarization.
In full agreement with the data described above, CCICR elicited by step depolarizations to +20 mV or higher in voltage-clamped myocytes had a relatively long latency (6.8 ±1.2 s measured to the onset of the foot preceding the fast rise of [Ca2+], n = 36 cells), thus further suggesting that, after the change of membrane potential and calcium-channel activation, several biochemical events obligatorily precede Ca2+ release and therefore that Ca2+ channels are not directly coupled to InsP3 or Ry receptors. This idea was strongly supported by experiments performed in cells treated with calyculin A and jasplakinolide, drugs that induce cytoskeletal reorganization and the formation of an actin ring separating the plasma membrane from the cytosolic organella (Hosoya et al., 1993; Patterson et al., 1999). Normally, VSMC actin filaments (Figure 5A, green) are homogeneously distributed and overlap the distribution of the SR (Figure 5A, red), which can appear near the plasma membrane. However, after incubation with calyculin A (Figure 5B, left) or jasplakinolide (Figure 5C, left), the SR seemed to be excluded from the vicinity of the membrane by a dense actin barrier. This cytoskeletal rearrangement maintained, however, either the CCICR induced by 70 mM external K+ or the ATP-dependent Ca2+ release unaltered (Figure 5B and C, right; see also Patterson et al., 1999). The amplitude of CCICR induced by 70 mM K+ in cells treated with calyculin A (0.11 ± 0.02 increase in fluorescence ratio, n = 11) or jasplakinolide (0.11 ± 0.01 increase in fluorescence ratio, n = 5) was not significantly different from that measured in control cells recorded in the same set of experiments (0.13 ± 0.02 increase in fluorescence ratio, n = 5).
Fig. 5. Cytoskeletal reorganization does not alter calcium-channel-induced Ca2+ release in arterial myocytes. (A) Example of depolarization (70 mM K+) and ATP (100 µM) induced Ca2+ release in a myocyte and confocal fluorescent staining of the cell with markers of the SR (antibodies against Ca ATPase, red) and cytoskeletal microfilaments (antibody against actin, green). (B), (C) Similar experiments as in (A) performed in cells subjected to prior incubation with calyculin A [(B) 100 nM, 30 min, n = 8 experiments] or jasplakinolide [(C) 1 µM, 120 min, n = 7 experiments]. Note in (B) and (C) the actin ring separating the plasma membrane and the cytosolic organella.
Ryanodine receptor activation is needed to evoke fast calcium-channel-induced Ca2+ release
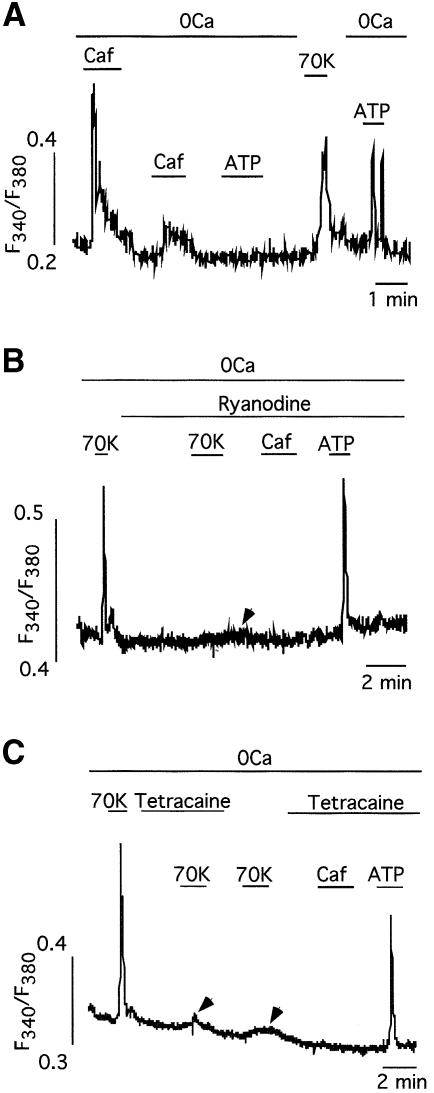
Ca2+ release from the SR in VSMCs is mediated by two separate sets of channels: InsP3 receptors, activated by ATP and other agonists, and Ry receptors, which can be pharmacologically activated by application of caffeine (Ehrlich and Watras, 1988; Blatter and Weir, 1992; Berridge, 1993; Pozzan et al., 1994). Repeated exposure of basilar arterial myocytes to caffeine resulted in depletion of the ATP-sensitive store (Figure 6A), thus suggesting that, as described for other cell types (Blatter and Weir, 1992; Khodakhah and Armstrong, 1997; Boittin et al., 1999), InsP3 receptors in basilar myocytes use the same functional Ca2+ pool as that utilized by Ry receptors. Using the appropriate concentration of externally applied Ry (20 µM in our dispersed myocyte preparation) to produce selective blockade of Ry receptors (Sutko et al., 1997), we could abolish the caffeine-induced Ca2+ release although the InsP3 receptor-dependent Ca2+-release signal induced by ATP was left intact (Figure 6B). Interestingly, in these conditions CCICR evoked by depolarization with 70 mM K+ was also almost completely blocked (97.5 ± 2.5% reduction of CCICR amplitude, n = 4 cells tested; Figure 6B), thus indicating that, besides InsP3-receptor activation, the generation of the CCICR signal also requires Ca2+ release through Ry receptors. This fact was confirmed by experiments performed with tetracaine, a membrane-permeant blocker of Ry receptors in smooth muscle (McCarron et al., 2002). In all cells tested (n = 10) tetracaine selectively suppressed the caffeine-induced Ca2+ release and inhibited by 86 ± 4.7% the fast CCICR signal evoked by depolarization with 70 mM K+, although the ATP-dependent Ca2+ signal was maintained (Figure 6C). In the presence of ryanodine or tetracaine a small slow CCICR signal was observed in numerous cells (arrows in Figure 6B and C). This signal, as well as the foot preceding CCICR in voltage-clamp cells, probably represents the initiatory InsP3-evoked calcium release due to depolarization when the Ry receptors are inhibited. These results strongly suggest that, although CCICR primarily requires activation of InsP3 receptors, stimulation of Ry receptors is necessary for the generation of the fast CCICR signal observed in arterial myocytes. It is most likely that, in basilar myocytes, Ca2+ released through InsP3 receptor channels activates Ry receptors to induce further Ca2+ release, as described for other VSMCs (Blatter and Weir, 1992; Boittin et al., 1999). Therefore the two Ca2+-release mechanisms interact to evoke full CCICR and myocyte contraction.
Fig. 6. Calcium-channel-induced Ca2+ release requires the activation of ryanodine receptors. (A) Depletion of the ATP-sensitive Ca2+ store by repeated application of caffeine (Caf, 5 mM). The response to ATP (100 µM) was restored after refilling the intracellular stores (n = 7 cells). (B) Application of ryanodine (20 µM) to the external solution blocks caffeine-induced Ca2+ release but maintains unaltered the Ca2+ release signal evoked by ATP (100 µM). Note that in cells treated with ryanodine, Ca2+ release induced by membrane depolarization (70K) is also strongly inhibited. The remaining slow signal (arrow) possibly represent the initiatory CCICR signal due to InsP3-dependent CCICR. (C) Abolishment of depolarization (70K)- and caffeine (5 mM) induced Ca2+ release by application of tetracaine (100 µM), a blocker of ryanodine receptors, to the external solution. In these experiments the Ca2+ release signal evoked by ATP (100 µM) was maintained. As in the middle panel, the arrows indicate slow CCICR signals resistant to tetracaine block.
Discussion
The results reported here describe a new mechanism of signaling InsP3 and Ry receptors in vascular smooth muscle, called CCICR, which can produce myocyte contraction. CCICR is initiated by the activation of voltage-gated Ca2+ channels which, after sensing membrane depolarization, trigger fast Ca2+-release from the SR in the absence of extracellular Ca2+ influx. CCICR can be triggered by L-type Ca2+-channel agonists and blocked by antagonists. CCICR requires stimulation of the G protein–phospholipase C pathway and activation of InsP3 receptors; however, a full CCICR is only seen if Ca2+ is also released through Ry receptors. Sequential activation of InsP3 and Ry receptors is probably achieved by spatial overlapping of both receptor types so that, as described before in VSMCs (Blatter and Weir, 1992; Boittin et al., 1999), Ca2+ released through InsP3-receptor channels activates Ry receptors to induce further Ca2+ release. This form of coupling VSMC electrical excitation to contraction is reminiscent of the classical mechanism existing in skeletal muscle where, after sensing membrane depolarization, dihydropyridine receptors directly activate Ry receptors of the SR to induce fast Ca2+ release and contraction (for review see Rios and Pizarro, 1991). In vascular myocytes, however, the coupling between membrane-voltage-gated Ca2+ channels and Ry receptors of the SR described here is not direct but involves G-protein activation and InsP3 production. It has recently been shown in a study performed on skeletal muscle cells that dihydropyridine receptors also mediate InsP3-dependent slow Ca2+ waves, occurring mainly in the nucleus, that regulate early gene expression (Jaimovich et al., 2000; Araya et al., 2003).
As well as in rat basilar myocytes (the preparation used in all the experiments described in this article), we have also observed CCICR in myocytes dispersed from mouse basilar artery and aorta as well as from pig coronary arteries. Hence it appears that CCICR is a general phenomenon, broadly represented among the various types of VSMCs. It has previously been reported in visceral smooth muscle (Suzuki and Hirst, 1999; van Helden et al., 2000) and vascular smooth muscle (Itoh et al., 1992; Ganitkevich and Isenberg, 1993) as well as in other cell types (Vergara et al., 1985; Kukuljan et al., 1994; Jaimovich et al., 2000; Mason and Mahaut-Smith, 2001) that membrane depolarization can increase InsP3 production and modulate agonist-induced Ca2+ release. In VSMCs of the rabbit mesentery artery it has been shown that hyperpolarization induced by pinacidil (an activator of KATP channels) reduces phosphatidylinositol hydrolysis and subsequent InsP3 production induced by norephinephrine. This effect of hyperpolarization is inhibited by high extracellular K+ (Itoh et al., 1992). Similarly, it has been reported in coronary myocytes that acetylcholine-induced InsP3-production or activation of phospholipase C by intracellular dialysis with GTP-γS are both potentiated by membrane depolarization (Ganitkevich and Isenberg, 1993). Therefore, the existence of a voltage-sensitive step in the InsP3 signaling pathway downstream from the surface membrane receptors (either at the level of G protein or phospholipase C) had been proposed (Itoh et al., 1992; Ganitkevich and Isenberg, 1993; Bolton et al., 1999; Suzuki and Hirst, 1999; Mason and Mahaut-Smith, 2001). Our results demonstrate that in arterial myocytes CCICR occurs without the presence of any stimulatory agonist or cell dialysis with GTP-γS, and they strongly suggest that CCICR depends on the sensing of membrane depolarization by Ca2+ channels and subsequent activation of G proteins. In the absence of membrane depolarization, CCICR can be triggered by Ca2+-channel agonists, thus indicating that sensitivity to membrane potential of the phosphatidylinositol cascade is not the primary cause of CCICR. Therefore, in cells having CCICR, voltage- and receptor-mediated pathways can independently increase InsP3 synthesis and Ca2+ release. It was already known that Ca2+ channels can be directly regulated by G proteins (Dolphin, 1998); however, the opposite phenomenon, regulation of G protein downstream of Ca2+-channel activation was unexpected. The mechanism whereby Ca2+ channels interact with G proteins appears to depend on the change of conformation of the voltage sensor and adjacent regions induced by membrane depolarization. These Ca2+-channel regions could possibly activate G protein by direct allosteric interactions, as it is known that membrane receptor, G proteins and L-type Ca2+ channels are in close proximity within membrane microdomains (Davare et al., 2001). Interestingly, suppression of Ca2+ influx by open-channel blockers like Cd2+ or Zn2+ does not prevent CCICR; however, this phenomenon is inhibited by Ca2+-channel gating modifiers such as diltiazem, D600 or nifedipine. The weak stimulatory effect of Bay K as compared with FPL to induce CCICR, as well as the differential potency of the Ca2+-channel antagonists to inhibit CCICR (diltiazem and D600 being more efficacious than nifedipine) is probably due to the fact that they interact with different regions of the L-type Ca2+-channel molecule. Ca2+-channel blockers of the benzothiazepine (diltiazem) and phenylalkylamine (D600) families share common molecular determinants with Ca2+-channel inactivation, thus explaining their potent inhibitory effect on CCICR (Hering et al., 1997, 1998; Bournaud et al., 1998; Kraus et al., 1998).
Finally, we have shown that CCICR is functionally relevant as rat basilar myocytes can be contracted in the absence of extracellular Ca2+. Therefore, in myocytes expressing CCICR, contraction due to membrane depolarization and extracellular Ca2+ influx is potentiated by Ca2+ release from the SR. These findings indicate that voltage-gated Ca2+ channels regulate vascular tone, not only permitting extracellular Ca2+ influx but also through modulation of Ca2+ release from the SR. They also explain previous reports (Somlyo and Somlyo, 1994; Kalsner, 1997; A.del Valle-Rodríguez, J.López-Barneo and J.Ureña, unpublished data) that some vascular territories contract without the need of external Ca2+. Abnormal activation of CCICR might participate in the production of vasospasms or transient increases in vascular resistance observed in pathological conditions (Sima et al., 1997). Since some Ca2+-channel gating modifiers, such as diltiazem and D600, modulate CCICR, the search of Ca2+-channel antagonists with a selective effect on CCICR should have therapeutic relevance.
Materials and methods
Cell preparation and treatments
Wistar rats (250–300 g) were deeply anesthetized and decapitated. Basilar arteries were dissected from the brain, cut into pieces and incubated at 4°C for 12–14 h in Hanks’ solution containing 1.3 mg/ml papain, 1.2 mg/ml collagenase, 0.2 mg/ml elastase and 0.75 mg/ml bovine serum albumin (BSA). After this time period cells were mechanically dispersed using a fire-polished Pasteur pipette and plated on pieces of coverslip. Cells were ready to use for the experiments within the next 2–3 h. For some experiments cells were incubated with either calyculin A (100 nM for 30 min) or jasplakinolide (1 µM for 2 h) before recording. These drugs were added to Hanks’ solution with 0.2 mg/ml BSA maintained at 37°C.
Cytosolic Ca2+ measurements and electrophysiological recordings
Cytosolic [Ca2+] was estimated in intact and dialyzed cells. In the first case, myocytes were incubated in Hanks’ solution with 1 µM Fura2-AM added for 15 min at room temperature. In dialyzed cells Fura2 potassium salt (50 µM) was added to the patch pipette solution. For the experiments a coverslip with cells was placed on a recording chamber (∼0.2 ml) mounted on the stage of an inverted microscope (Axiovert 35, Zeiss) equipped with epifluorescence and photometry. For fluorescence excitation, we used a monochromator (Polycrome IV, T.I.L.L., Photonics) and the light was deflected by a dichroic mirror (BSP 430 nm) into the microscope objective (Plan-Neofluar 40×; NA 0.75, Zeiss). Fluorescence from the myocytes was filtered twice, first by a Zeiss LP470 filter and then by a short-pass filter (540 SP, Zeiss), and detected by a CCD camera (C4880–80, Hamamatsu). The software used was AquaCosmos 2.0 (Hamamatsu). Alternating excitation wavelengths of 340 and 380 nm were used and background fluorescence was subtracted before obtaining the F340/F380 ratio. In the text and figures, variations of cytosolic [Ca2+] are given as changes of fluorescence ratio. Voltage- and current-clamp recordings were obtained using the whole-cell configuration of the patch–clamp technique. Details of our experimental setup have been given previously (Franco-Obregón et al., 1995). We used an EPC-7 patch–clamp amplifier (List Electronics) and electrodes with resistance between 2 and 5 MΩ. When cytosolic [Ca2+] and membrane-holding potential or current were recorded simultaneously, the signals were digitized at a sampling interval of 500 ms and filtered at 3 kHz. All the experiments were performed at room temperature (∼22°C).
Confocal microscopy and immunocytochemistry
To study myocyte contraction in parallel with cytosolic [Ca2+], we used a confocal microscope (Leica, TCS SP2). Cells were loaded with 1 µM Fluo3-AM following the same protocol as indicated above for Fura2-AM. Excitation was done with an argon–krypton laser (488 nm) and the emitted fluorescence (500–600 nm) was recorded through an oil immersion objective (40 × 1.25). An image (512 × 512 pixels) was acquired every 2 s. Immunocytochemical studies were done on fixed cells following standard protocols. Briefly, isolated cells were placed on coverslips and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) (137 mM NaCl, 2.68 mM KCl, 4.02 mM Na2HPO4, 1.76 mM KH2PO4) for 20 min. Subsequently, cells were rinsed in PBS and incubated for 60 min in non-specific binding solution [PBS, 3% fetal bovine serum (FBS), 0.1% Triton X-100]. We used anti-SERCA-2 antibody (ABR-MA3-910, Alexis) and donkey antimouse Texas red (715-075-150, Jackson) secondary antibody to stain the SR. Muscle actin was stained with a monoclonal antibody fluorescein isothiocyanate (FITC) conjugate (F3777, Sigma Aldrich). Cells were incubated overnight at 4°C in mouse anti-SERCA-2 diluted 1:1000 in PBS, 3% FBS and 0.1% Triton X-100. Cells were rinsed in PBS and incubated for 60 min in donkey antimouse Texas red diluted 1:500. Coverslips were rinsed in PBS and incubated overnight at 4°C in mouse anti-α-actin FITC diluted 1:1000. Finally coverslips were rinsed in PBS and mounted on slides with DPX. Fluorescence was observed with the confocal microscope.
Solutions
The composition of Hanks’ solution was 125 mM NaCl, 5.36 mM KCl, 5 mM NaHCO3, 0.34 mM Na2HPO4, 0.44 mM KH2PO4, 10 mM glucose, 1.45 mM sucrose and 10 mM HEPES pH 7.4. During the experiments the recording chamber was continuously superfused with a control external solution containing 140 mM NaCl, 2.7 mM KCl, 2.5 mM CaCl2 1 mM MgCl2, 10 mM HEPES pH 7.4. Unless otherwise noted, all the drugs used were added to this solution at the indicated concentration. The 70K solution was obtained by replacing 70 mM of NaCl with KCl. The 0Ca solution was obtained by substituting Mg2+ for Ca2+ and adding 2 mM EGTA. Patch–clamp experiments were done by dialyzing the cells with an internal solution containing 83 mM K glutamate, 47 mM KCl, 1 mM MgCl2, 10 mM HEPES 4 mM ATP-Mg, 50 µM Fura2-potassium salt pH 7.2. To record whole-cell calcium currents the solutions used were as follows: external (140 mM NaCl, 2.7 mM KCl, 10 mM BaCl2, 10 mM HEPES, pH 7.4); internal (100 mM CsCl, 30 mM CsF, 2 mM MgCl2, 4 mM ATP-Mg, 10 mM EGTA, 10 mM HEPES pH 7.2).
Data analysis
Data are expressed as mean ± SE and the statistical significance was estimated using Student’s t test. Values of p < 0.05 were considered significant. Normalized calcium conductance was estimated by measuring the amplitude of the repolarizing tail current pulses. The data points relating calcium conductance or fluorescence ratio to membrane potential were least squares fitted to a Boltzmann function of the form
where Vm is the membrane potential during each pulse, V1/2 is the potential at which 50% of maximal amplitude is obtained and k is a slope factor that indicates the steepness of the curve.
Acknowledgments
Acknowledgements
We are indebted to Dr Konstantin Levitsky for help with the confocal microscope and to Dr Manuel Carrasco and Dr Juanjo Toledo-Aral for technical advice and comments on the manuscript. A.V.-R. is the recipient of a fellowship from FIS. Research was supported by grants from the Spanish Ministry of Science and Technology (1FD97-1614 and PM99-0120). J.L.-B. received the ‘Ayuda a la investigación 2000’ from the Juan March Foundation.
References
- Araya R., Liberona,J.L., Cardenas,J.C., Riveros,N., Estrada,M., Powell,J.A., Carrasco,M.A. and Jaimovich,E. (2003) Dihydro pyridine receptors as voltage sensors for a depolarization-evoked, IP3R-mediated, slow calcium signal in skeletal muscle cells. J. Gen. Physiol., 121, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B.P., Sturek,M., Puga,A. and Hermsmeyer,K. (1986) Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ. Res., 59, 229–235. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. (1993) Inositol trisphosphate and calcium signalling. Nature, 361, 315–325. [DOI] [PubMed] [Google Scholar]
- Blatter L.A. and Weir,W.G. (1992) Agonist-induced [Ca2+]i waves and Ca2+-induced Ca2+ release in mammalian vascular smooth muscle cells. Am. J. Physiol., 263, H576–H586. [DOI] [PubMed] [Google Scholar]
- Boittin F.X., Macrez,N., Halet,G. and Mironneau,J. (1999) Norepinephrine-induced Ca2+ waves depend on InsP3 and ryanodine receptor activation in vascular myocytes. Am. J. Physiol., 277, C139–C151. [DOI] [PubMed] [Google Scholar]
- Bolton T.B., Prestwich,S.A., Zholos,A.V. and Gordienko,D.V. (1999) Excitation–contraction coupling in gastrointestinal and other smooth muscles. Annu. Rev. Physiol., 61, 85–115. [DOI] [PubMed] [Google Scholar]
- Bournaud R., Hidalgo,J., Melliti,K. and Shimahara,T. (1998) The action of diltiazem and gallopamil (D600) on calcium channel current and charge movement in mouse Purkinje neurons. Neurosci. Lett., 241, 163–166. [DOI] [PubMed] [Google Scholar]
- Davare M.A., Avdonin,V., Hall,D.D., Peden,E.M., Burette,A., Weinberg,R.J., Horne,M.C., Hoshi,T. and Hell,J.W. (2001). A β2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav 1.2. Science, 293, 98–101. [DOI] [PubMed] [Google Scholar]
- Dolphin A.C. (1998) Mechanisms of modulation of voltage-dependent calcium channels by G proteins. J. Physiol., 506, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich B.E. and Watras,J. (1988) Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature, 336, 583–586. [DOI] [PubMed] [Google Scholar]
- Franco-Obregón A., Ureña,J. and Lopez-Barneo,J. (1995) Oxygen-sensitive calcium channels in vascular smooth muscle and their possible role in hypoxic arterial relaxation. Proc. Natl Acad. Sci. USA, 92, 4715–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich V.Ya. and Isenberg,G. (1993) Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. J. Physiol., 470, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R.W. and Lederer,W.J. (1991) Properties of L-type calcium channel gating current in isolated guinea pig ventricular myocytes. J. Gen. Physiol., 98, 265–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering S., Aczel,S., Kraus,R.L., Berjukow,S., Striessnig,J. and Timin,E.N. (1997) Molecular mechanism of use-dependent calcium channel block by phenylalkylamines: Role of inactivation. Proc. Natl Acad. Sci. USA, 94, 13323–13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering S., Berjukow,S., Aczel,S. and Timin,E.N. (1998) Calcium channel block and inactivation: common molecular determinants. Trends Pharmacol. Sci., 19, 439–443. [DOI] [PubMed] [Google Scholar]
- Hosoya N., Mitsui,M., Yazama,F., Ishihara,H., Ozaki,H., Karaki,H., Hartshorne,D.J. and Mohri,H. (1993) Changes in the cytoskeletal structure of cultured smooth muscle cells induced by calyculin-A. J. Cell Sci., 105, 883–890. [DOI] [PubMed] [Google Scholar]
- Itoh T., Seki,N., Suzuki,S., Ito,S., Kajikuri,J. and Kuriyama,H. (1992) Membrane hyperpolarization inhibits agonist-induced synthesis of inositol 1,4,5-trisphosphate in rabbit mesenteric artery. J. Physiol., 451, 307–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimovich E., Reyes,R., Liberona,J.L. and Powell,J.A. (2000) IP3 receptors, IP3 transients, and nucleus-associated calcium signals in cultured skeletal muscle. Am. J. Physiol., 278, C998–C1010. [DOI] [PubMed] [Google Scholar]
- Kalsner S. (1997) Vasodilator action of calcium antagonists in coronary arteries in vitro. J. Pharmacol. Exp.Ther., 281, 634–642. [PubMed] [Google Scholar]
- Khodakhah K. and Armstrong,C.M. (1997) Inositol trisphosphate and ryanodine receptors share a common functional Ca2+ pool in cerebellar Purkinje neurons. Biophys. J., 73, 3349–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kanaide,H. and Nakamura,M. (1986) Complete overlap of caffeine- and K+ depolarization-sensitive intracellular calcium storage site in cultured rat arterial smooth muscle cells. J. Biol. Chem., 261, 15709–15713. [PubMed] [Google Scholar]
- Kraus R.L., Hering,S., Grabner,M., Ostler,D. and Striessnig,J. (1998) Molecular mechanism of diltiazem interaction with L-type Ca2+ channels. J. Biol. Chem., 273, 27205–27212. [DOI] [PubMed] [Google Scholar]
- Kuga T., Sadoshima,J., Tomoike,H., Kanaide,H., Akaike,N. and Nakamura,M. (1990) Actions of Ca2+ antagonists on two types of Ca2+ channels in rat aorta smooth muscle cells in primary culture. Circ. Res., 67, 469–480. [DOI] [PubMed] [Google Scholar]
- Kukuljan M., Rojas,E., Catt,K.J. and Stojilkovic,S.S. (1994) Membrane potential regulates inositol 1,4,5-trisphosphate-controlled cytoplasmic Ca2+ oscillations in pituitary gonadotrophs. J. Biol. Chem., 269, 4860–4865. [PubMed] [Google Scholar]
- McCarron J.G., Craig,J.W., Bradley,K.N. and Muir,T.C. (2002) Agonist-induced phasic and tonic responses in smooth muscle are mediated by InsP3. J. Cell Sci., 115, 2207–2218. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Kanaji,T., Nakade,S., Kanno,T. and Mikoshiba,K. (1997) 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem., 122, 498–505. [DOI] [PubMed] [Google Scholar]
- Mason M.J. and Mahaut-Smith,M.P. (2001) Voltage-dependent Ca2+ release in rat megakaryocytes requires functional IP3 receptors. J. Physiol., 533, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M.T., Cheng,H., Rubart,M., Santana,L.F., Bonev,A.D., Knot,H.J. and Lederer,W.J. (1995) Relaxation of arterial smooth muscle by calcium sparks. Science, 270, 633–637. [DOI] [PubMed] [Google Scholar]
- Patterson R.L., van Rossum,D.B. and Gill,D.L. (1999) Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell, 98, 487–499. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto,R., Volpe,P. and Meldolesi,J. (1994) Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev., 74, 595–636. [DOI] [PubMed] [Google Scholar]
- Rios E. and Pizarro,G. (1991) Voltage sensor of excitation–contraction coupling in skeletal muscle. Physiol. Rev., 71, 849–908. [DOI] [PubMed] [Google Scholar]
- Sima B., Weir,B.K., Macdonald,R.L. and Zhang,H. (1997) Extracellular nucleotide-induced [Ca2+]i elevation in rat basilar smooth muscle cells. Stroke, 28, 2053–2058. [DOI] [PubMed] [Google Scholar]
- Smani T., Iwabuchi,S., Lopez-Barneo,J. and Ureña,J. (2001) Differential segmental activation of Ca2+ -dependent Cl– and K+ channels in pulmonary arterial myocytes. Cell Calcium, 29, 369–377. [DOI] [PubMed] [Google Scholar]
- Smith R.J., Sam,L.M., Justen,J.M., Bundy,G.L., Bala,G.A. and Bleasdale,J.E. (1990) Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J. Pharmacol. Exp. Ther., 253, 688–697. [PubMed] [Google Scholar]
- Somlyo A.P. and Somlyo,A.V. (1994) Signal transduction and regulation in smooth muscle. Nature, 372, 231–236. [DOI] [PubMed] [Google Scholar]
- Sutko J.L., Airey,J.A., Welch,W. and Ruest,L. (1997) The pharmacology of ryanodine and related compounds. Pharmacol. Rev., 49, 53–98. [PubMed] [Google Scholar]
- Suzuki H. and Hirst,G.D. (1999) Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J. Physiol., 517, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen,P.J., Drobak,B.K., Hanley,M.R. and Dawson,A.P. (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc. Natl Acad. Sci. USA, 87, 2466–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden D.F., Imtiaz,M.S., Nurgaliyeva,K., von der Weid,P. and Dosen,P.J. (2000) Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J. Physiol., 524, 245–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara J., Tsien,R.Y. and Delay,M. (1985) Inositol 1,4,5-trisphosphate: a possible chemical link in excitation–contraction coupling in muscle. Proc. Natl Acad. Sci. USA, 82, 6352–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Rampe,D. and Triggle,D.J. (1991) Pharmacological, radioligand binding and electrophysiological characteristics of FPL 64176, a novel nondihydropyridine Ca2+ channel activator, in cardiac and vascular preparations. Mol. Pharmacol., 40, 734–741. [PubMed] [Google Scholar]