Abstract
The c-Myb transcription factor is expressed in immature haemopoietic cells and at key stages during differentiation. Loss of the c-myb gene results in embryonic lethality because mature blood cells fail to develop, although commitment to definitive haemopoiesis occurs. We have generated a knockdown allele of c-myb, expressing low levels of the protein, which has enabled us to investigate further the involvement of c-Myb in haemopoiesis. Low levels of c-Myb are sufficient to allow progenitor expansion but, importantly, we show that progression of progenitors towards terminal differentiation is significantly altered. Suboptimal levels of c-Myb favour differentiation of macrophage and megakaryocytes, while higher levels seem to be particularly important in the control of erythropoiesis and lymphopoiesis. We provide evidence that the transition from the CFU-E to erythroblasts is critically dependent on c-Myb levels. During thymopoiesis, c-Myb appears to regulate immature cell numbers and differentiation prior to expression of CD4 and CD8. Overall, our results point to a complex involvement of c-Myb in the regulation of proliferation and commitment within the haemopoietic hierarchy.
Keywords: c-Myb/commitment/Cre-loxP/haemopoietic progenitors/knockdown mutation
Introduction
Three members of the Myb family of transcriptional regulatory proteins (A-, B- and c-Myb) are expressed in vertebrates and appear to have roles in proliferation, apoptosis and differentiation (Oh and Reddy, 1999). c-Myb is expressed in a variety of tissues during development and in the adult (Sitzmann et al., 1995; Ess et al., 1999), and considerable evidence points to a crucial role in haemopoiesis. Hence, c-myb is highly expressed in immature haemopoietic progenitors (Kastan et al., 1989) and can be detected at sites of definitive haemopoietic stem cell emergence in the para-aortic splanchnopleura (Labastie et al., 1998) and at the onset of definitive haemopoietic activity in the yolk sac (Palis et al., 1999).
The pattern of c-myb RNA expression in haemopoietic cells is reflected by the fact that mice homozygous for an inactivated allele of c-myb die in utero at ∼E15 of anaemia due to a failure to develop fetal liver haemopoiesis (Mucenski et al., 1991). Yolk sac haemopoiesis is apparently unaffected by the absence of c-Myb since primitive nucleated erythrocytes are present (Mucenski et al., 1991), and are also produced during in vitro differentiation of c-myb–/– embryonic stem (ES) cells (Clarke et al., 2000). Apart from primitive erythrocytes, the only mature cells present in the c-myb–/– fetal liver are megakaryocytes and macrophages. It has been suggested that this haemopoietic failure might be accounted for by a lack of stem cell specification in the aorta–gonad–mesonephros (AGM) region of c-myb–/– embryos (Mukouyama et al., 1999). However, examination of the fetal liver from c-myb–/– embryos and analysis of cells arising during the in vitro differentiation of c-myb–/– ES cells revealed that cells with a progenitor phenotype are produced in the absence of c-Myb. Hence, cells co-expressing the progenitor antigen CD34 and the pan-haemopoietic marker CD45 were detected in the c-myb–/– fetal liver (Sumner et al., 2000) and amongst the cells derived from c-myb–/– ES cells (Clarke et al., 2000). These cells did not possess functional activity as progenitors when assayed for colony formation in vitro. The study by Sumner et al. (2000) also showed that c-myb–/– ES cells introduced into wild-type blastocysts became part of a chimeric animal contributing widely to non-haemopoietic tissues although the presence of c-myb–/– haemopoietic cells was very restricted. In this latter study no contribution of c-myb–/– cells to adult haemopoiesis could be detected but examination of the fetal liver of E11–12 chimeras revealed the presence of c-myb–/– progenitor-like cells that subsequently failed to expand or were lost by terminal differentiation. A related study involving chimeric animals suggested that some c-myb–/– haemopoietic progenitors could be detected in the adult. Allen et al. (1999) were able to identify a small number of very immature c-myb–/– thymocytes in the adult thymus of chimeras between c-myb–/– ES cells and rag-1–/– host blastocysts. The highly selective environment used in this latter study is the likely explanation for the existence of detectable c-myb–/– haemopoietic cells; nevertheless, as with the study by Sumner et al. (2000), their presence is indicative of the generation of definitive haemopoietic cells. Whether these cells are the descendants of haemapoietic stem cells (HSCs) originating in the AGM or from some later haemogenic endothelium is not known. Certainly, haemogenic sites within the AGM appear to be functioning in c-myb–/– embryos since haemopoietic cells could be detected emerging from the endothelial layer with the same frequency in the wild type and knockout (N.Emambokus and J.Frampton, unpublished data).
Here we describe a knockdown allele of c-myb, which expresses 5–10% of the wild-type level of c-Myb. Analysis of embryos carrying this allele shows that in the presence of lower than normal amounts of c-Myb, haemopoietic progenitors are generated in the same numbers as in the wild type, but their differentiation potentials are severely affected. Our study extends our knowledge of the role of c-Myb in haemopoiesis showing that, although it is not required for the establishment of definitive haemopoiesis, it is a crucial player in the achievement of the full capacity of the system. We propose that this key role for c-Myb involves regulation both of progenitor maintenance, by an influence on expansion versus commitment, and of key points along specific pathways of differentiation.
Results
Generation of a conditional allele of c-myb
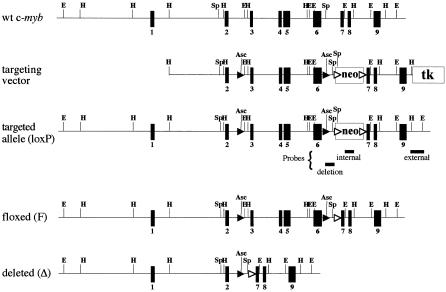
In order to create a conditional floxed allele of c-myb, we introduced loxP sites upstream of exon 3 and downstream of exon 6 encompassing the DNA binding domain coding sequences (Figure 1). A neoR cassette flanked by Flp recombinase recognition sites (FRT) was cloned into intron 6 for positive selection while herpes simplex virus (HSV)-1 tk sequences enabled negative selection. The resulting targetting vector was introduced into ES cells. Clones were screened using a probe external to the targetting sequences and an internal neoR coding sequence probe (Figure 1). Of 23 correctly targeted ES clones 12 were screened by PCR to identify the presence of the two loxP sites. Six clones were found to contain both loxP sites. Clones 2C8 and 2J7 were used to generate chimeras, which transmitted the targeted allele (designated c-mybloxP) through the germline.
Fig. 1. Generation of a conditional c-myb allele. The targetting vector is shown together with the organization of the wild-type c-myb gene. A neomycin resistance cassette (neo) for positive selection and the HSV-1 thymidine kinase gene (tk) for negative selection were introduced into intron 6 and just downstream of exon 9 respectively. The neo cassette was flanked by Flp recognition sites (open arrowheads). LoxP sites (filled arrowheads) were introduced into introns 2 and 6. The vertical black boxes represent exons. Relevant restriction endonuclease sites are indicated (E, EcoRI; H, HindIII and Sp, SpeI). The fragments used to probe Southern blots are indicated (external, neo and deletion probes).
To determine whether the c-mybloxP allele could be deleted in vivo, c-myb+/loxP animals were crossed with mice that carry the widely expressed GATA-Cre transgene (Jasinski et al., 2001). Deletion was demonstrated by PCR analysis of tail DNA, and subsequent intercrossing of the c-myb+/Δ progeny revealed that c-mybΔ/Δ embryos were indistinguishable from c-myb–/– as originally described by Mucenski et al. (1991) (data not shown).
We crossed c-myb+/loxP animals with Flpe transgene mice (Dymecki, 1996) to bring about deletion of the FRT-flanked sequences. Deletion of neoR was detected by PCR and confirmed by Southern blotting. Intercrossing of mice carrying the floxed allele lacking neoR (c-myb+/F) demonstrated that homozygotes reach adulthood at the expected frequency and with no obvious phenotypic difference compared with wild-type animals (data not shown).
Insertion of the neoR cassette into intron 6 of the c-myb gene results in a knockdown allele that affects haemopoietic development
In breeding animals carrying the c-mybloxP allele we mated c-myb+/loxP and c-myb+/– mice. To our surprise there was an apparent absence of c-myb–/loxP offspring (Table I). This immediately suggested that the c-mybloxP allele is completely or partially disabled, most likely as a result of the insertion of the neoR cassette. To confirm this we set up matings between c-myb+/– and c-myb+/F mice and, as expected, found that c-myb–/F offspring were born with a normal Mendelian frequency (Table I).
Table I. c-myb floxed and null allele crosses (live births).
| c-myb+/loxP × c-myb+/– (n = 41) | ||||
| c-myb+/+ | c-myb+/– | c-myb+/loxP | c-myb–/loxP | |
| |
19 |
11 |
11 |
0 |
| c-myb+/F × c-myb+/– (n = 25) | ||||
| c-myb+/+ | c-myb+/– | c-myb+/F | c-myb–/F | |
| 7 | 5 | 6 | 7 | |
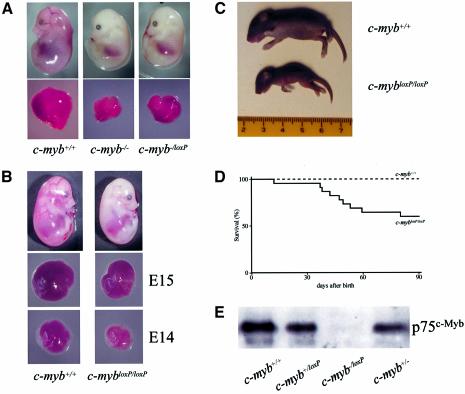
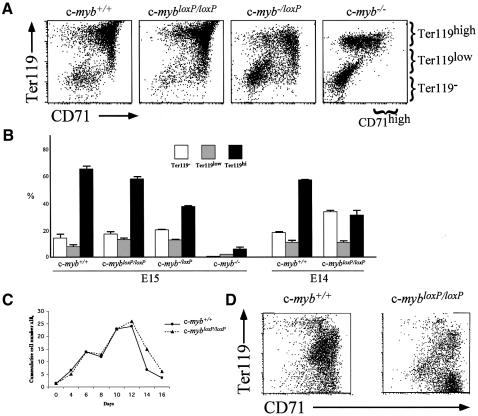
The absence of c-myb–/loxP neonates or adults implied that embryos were lost in utero. Examination of litters from c-myb–/loxP × c-myb+/– matings revealed that c-myb–/loxP embryos die at ∼E16. Interestingly, we noticed that the phenotype of these embryos was slightly different compared with c-myb–/– embryos. Although clearly anaemic with a small liver, E15 c-myb–/loxP embryos had a larger fetal liver compared with c-myb–/– (Figure 2A). This suggested that the c-mybloxP and c-myb– alleles are not equivalent. E15 embryos with a c-mybloxP/loxP genotype also exhibited an apparently anaemic phenotype, however, this appeared to be less severe than in c-myb–/loxPembryos with little or no reduction in fetal liver cellularity (Figure 2B and Table II), although just one day earlier at E14 the c-mybloxP/loxP fetal liver was more obviously deficient (Figure 2B, lower panels). This apparent ‘recovery’ of fetal liver haemopoiesis between E14 and E15 is reflected in the birth of pups with a c-mybloxP/loxP genotype. Although smaller compared to wild-type siblings (Figure 2C), c-mybloxP/loxP animals reached adulthood (Table III), ∼60% surviving beyond 3 months (Figure 2D). Premature death of c-mybloxP/loxP adults seemed to occur as the result of a variety of haematological deficiencies including anaemia and immune dysfunction possibly leading to increased tumour frequency and autoimmune diseases (data not shown). These disease phenotypes are currently being investigated in more detail.
Fig. 2. Insertion of the neoR cassette into exon 6 of the c-myb gene results in a knockdown allele that affects development. (A) Wild-type, c-myb–/– and c-myb–/loxP E15 embryos and dissected fetal livers. (B) Wild-type, c-mybloxP/loxP and c-myb–/loxP E15 embryos and dissected fetal livers from E15 and E14 embryos. (C) Wild-type and c-mybloxP/loxP littermates 6 days after birth. (D) c-mybloxP/loxP mice have a reduced lifespan. The graph represents the survival of 23 wild-type compared with 23 c-mybloxP/loxP mice derived from 15 litters. (E) The c-mybloxP allele produces low levels of full-length c-Myb protein. Western blot analysis of protein extracts of fetal livers from E11 embryos probed with a c-Myb specific monoclonal antibody. Ponceau staining of the filter immediately after transfer indicated that all tracks contained equivalent amounts of protein.
Table II. Fetal liver and thymus cellularity in c-myb knockdown embryos.
| Genotype | Fetal liver (× 10–7) |
Fetal thymus (× 10–4) | |
|---|---|---|---|
| E14 | E15 | E14 | |
| c-myb+/+ | 1.43 ± 0.56 | 3.46 ± 0.53 | 2.15 ± 0.9 |
| c-myb+/– | 1.10 ± 0.11 | ND | 2.7 ± 0.3 |
| c-myb+/loxP | 1.38 ± 0.55 | ND | ND |
| c-myb–/loxP | 0.14 ± 0.0 | ND | 0.2 ± 0.0 |
| c-mybloxP/loxP | 0.43 ± 0.15 | 3.15 ± 0.46 | 0.2 ± 0.07 |
Table III. Frequency of c-mybloxP/loxP live births.
| Genotype | Expected number of embryos | Observed number of embryos |
|---|---|---|
| c-myb+/+ | 23 | 22 |
| c-myb+/loxP | 46 | 51 |
| c-mybloxP/loxP | 23 | 19 |
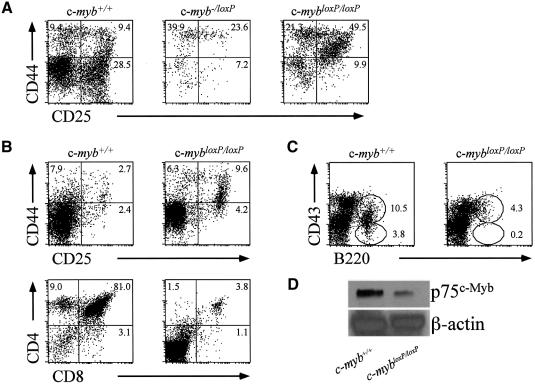
The most likely explanation for these observations is that the c-mybloxP allele is expressed, but at a much lower level than the wild-type gene. In order to test this, western blot analysis was performed on fetal liver cells from E11 embryos, at a stage at which phenotypic differences were found to be minimal among the different genotypes (data not shown). Probing with an anti-c-Myb antibody revealed the presence of p75c-Myb in all samples derived from embryos with various combinations of wild-type, c-mybloxP and c-myb– alleles (Figure 2E). Possession of one null or c-mybloxP allele (c-myb+/– or c-myb+/loxP) reduced the amount of c-Myb to about half compared with the wild type (c-myb+/+). Although faint, full-length c-Myb was clearly detected in c-myb–/loxP embryos at a level ∼5% of that of the wild type. Additional evidence from CD4+8+ thymocytes that the c-mybloxP allele produces reduced levels of c-Myb is presented in Figure 5D.
Fig. 5. Lymphoid differentiation is particularly sensitive to the level of c-Myb. (A) Flow cytometric analysis of fetal thymus cells from wild-type, c-mybloxP/loxP and c-myb–/loxP E15 embryos stained with anti-CD44–PE and anti-CD25–FITC. (B) Flow cytometric analysis of thymus cells from wild-type and c-mybloxP/loxP neonates stained with anti-CD44–PE and anti-CD25–FITC (upper panels) and anti-CD4–PE and anti-CD8–FITC (lower panels). (C) Flow cytometric analysis of bone marrow cells from wild-type and c-mybloxP/loxP neonates stained with anti-CD43–PE and anti-B220–FITC. (D) Western blot analysis of protein extracts of sorted CD4+8+ 3 month-old thymocytes probed with monoclonal antibodies specific for c-Myb and β-actin as a control for loading.
Taken together with the fact that the null allele produces no full-length c-Myb (Mucenski et al., 1991) these results demonstrate that the insertion of the neoR cassette produces an allele of c-myb which generates ∼5–10% of the wild-type level of c-Myb.
Low levels of c-Myb permit haemopoietic progenitor development and expansion but lead to embryonic lethality
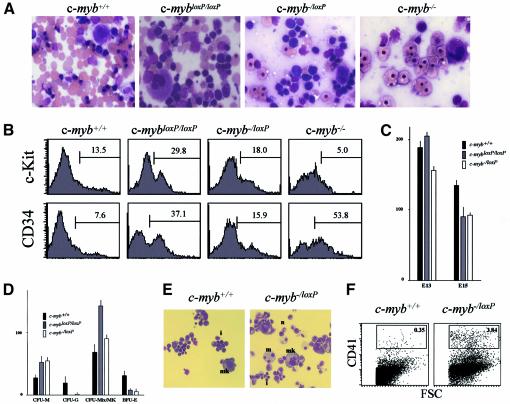
The gross morphology of the fetal livers of c-myb–/loxP and c-mybloxP/loxP embryos indicated the presence of more cells compared with c-myb–/– embryos. Touch preparations of fetal livers from E15 were stained with May–Grünwald–Giemsa and indicated distinct cellular profiles in c-myb–/loxP and c-mybloxP/loxP embryos (Figure 3A). Although c-myb–/loxP fetal liver, like that from c-myb–/– embryos, contained primitive erythrocytes, mature megakaryocytes but no definitive enucleated erythrocytes (compare with Mucenski et al., 1991; Sumner et al., 2000) there were greater numbers of dark staining cells with an immature morphology. The situation in the c-mybloxP/loxP embryos was intermediate between c-myb–/loxP and wild type in that, in addition to increased numbers of progenitor-like cells, a greater number of differentiated cells, including enucleated erythrocytes were apparent (Figure 3A). Flow cytometric analysis of E14 cells stained with antibodies specific for characteristic progenitor antigens confirmed that the c-mybloxP/loxP and c-myb–/loxP fetal livers contained a dramatically increased proportion of immature cells. Hence, in the fetal liver CD34 and c-Kit were expressed on 37.1 and 29.8% of c-mybloxP/loxP cells and on 15.9 and 18% of c-myb–/loxP cells, compared with 7.6 and 13.5% in the wild type (Figure 3B). As we have previously described, c-myb–/– fetal liver contains no bright c-Kit+ cells but CD34+ cells are present, roughly half being CD45+ (Sumner et al., 2000).
Fig. 3. Low levels of c-Myb permit haemopoietic progenitor development but affect commitment and differentiation. (A) The c-myb genotype affects fetal liver cellularity. Touch preparations of E15 fetal livers were stained with May–Grünwald–Giemsa. (B) Low level c-Myb expression leads to a relative accumulation of progenitor cells. Flow cytometric analysis of fetal liver cells from wild-type, c-mybloxP/loxP, c-myb–/loxP and c-myb–/– E14 embryos stained with anti-c-Kit–PE (upper panels) or anti-CD34–biotin followed by streptavidin–PE (lower panels). The bar indicates the percentage of positive cells relative to staining obtained using an isotype control. (C and D) Progenitors are functional in the presence of lower than normal levels of c-Myb. Fetal liver cells (4 × 104) from E13 or E15 embryos were plated in duplicate in methycellulose to allow growth and identification of all myeloid types. Each determination was repeated at least three times. The data is presented as the total colony number (C) for E13 or E15 derived fetal liver and as the number of individual colony types obtained from E13 cells (D). (E) Mixed colonies differentiate more rapidly from c-myb–/loxP fetal liver progenitors. Cytospins of day 4 colonies from a CFU assay of E13 fetal livers were stained with May-Grünwald–Giemsa. Typical examples of macrophage (m), megakaryocytes (mk) and neutrophils (n) as well as more immature cells (i) are indicated. (F) Megakaryopoiesis is increased in vivo. Flow cytometric analysis of fetal liver cells from wild-type and c-myb–/loxP E13 embryos stained with anti-CD41 followed by streptavidin–PE and anti-IgG1–FITC.
Our previous studies had shown that the definitive CD34+ progenitor-like cells produced in the absence of c-Myb have no capacity to proliferate (Clarke et al., 2000; Sumner et al., 2000). The higher numbers of cells with an immature phenotype in embryos possessing one or two copies of the knockdown c-mybloxP allele suggested that the progenitors might have some proliferative capacity. To test this we set up in vitro assays for colony forming units (CFUs) in methylcellulose containing growth factors that would allow identification of all multi- and monopotent myeloid progenitors. Such assays were performed using cells derived from fetal liver at E13 and E15 of wild-type, c-mybloxP/loxP and c-myb–/loxP embryos. The results of these assays are in striking contrast to those obtained when c-myb–/– fetal liver was examined in this way (Mucenski et al., 1991; Sumner et al., 2000). Firstly, cells within the fetal livers of both c-mybloxP/loxP and c-myb–/loxP embryos possess in vitro colony forming capacity in contrast to the situation in c-myb–/– fetal livers. Moreover, similar cumulative numbers of CFUs were obtained from the three genotypes at E13, with some decrease in numbers for c-mybloxP/loxP and c-myb–/loxP relative to the wild type at E15 (Figure 3C). Secondly, colony morphology and the distribution of CFU types was dramatically altered in the presence of one or two copies of the c-mybloxP allele. Hence, there was little sign of granulocytes either as monolineage CFU-G or in bipotent CFU-GM, and erythroid progenitors (BFU-E) were also significantly reduced in number (Figure 3D). In contrast, there was an ∼2-fold increase in the number of monocyte/macrophage colonies (CFU-M), although by E15 there appeared to be a drop off in this number in c-myb–/loxP relative to the wild type (Figure 3D and data not shown). Most dramatic, was the increase in colonies containing a high proportion of megakaryocytes (CFU-mix/MK). Many of these colonies were very large compared with those from wild-type fetal livers and staining with May–Grünwald–Giemsa revealed that a majority of cells were megakaryocytes and macrophages, although other cells, including neutrophils, were present (data not shown). Terminally differentiated cells appeared in the c-myb–/loxP mixed colonies at a relatively early stage of the assay compared with those from wild-type fetal liver (Figure 3E). Consistent with the increased number of CFUs containing megakaryocytes we also found much higher levels of megakaryocytes in the fetal liver of c-myb–/loxP relative to wild-type embryos. Hence, the proportion of CD41high cells was 3.84% in the mutant compared with 0.35% in wild type at E13 (Figure 3F).
Taken together, these data show that progenitor numbers are not substantially affected by lower levels of c-Myb, but that their subsequent commitment and differentiation potential is altered. The presence of increased numbers of megakaryocyte-containing colonies, often with an aberrant phenotype, in conjunction with the absence of definable granulocytic and erythroid progenitors, suggests that lower levels of c-Myb either favour some differentiation routes or lead to selective loss of specific differentiated cell types.
Erythroid differentiation is perturbed in the presence of reduced levels of c-Myb
To address whether the lack of definitive erythrocytes in c-myb–/loxP embryos and the absence of BFU-E in colony assays of c-mybloxP/loxP and c-myb–/loxP fetal liver cells reflected that erythroid progenitors never existed or was the result of aberrant maintenance or differentiation of these progenitors, we next looked at the status of erythropoiesis. Cells from wild-type, c-mybloxP/loxP, c-myb–/loxP and c-myb–/– E15 fetal livers were simultaneously stained for transferrin receptor (CD71) and the erythroid antigen Ter119 (Socolovsky et al., 2001). In c-myb–/– fetal liver this showed the expected complete absence of all cells from the CFU-E stage (CD71high Ter119–) through to late erythroblasts (CD71high Ter119high) and the presence of differentiated red cells (CD71–Ter119high), which must be primitive erythrocytes based on the May–Grünwald–Giemsa stained preparations (Figures 3A and 4A and data not shown). In contrast, although only primitive erythrocytes were observed in touch preparations of c-myb–/loxP fetal liver (Figure 3A), the flow cytometric analysis showed that erythroid precursors were present (Figure 4A). The ratio of early to late erythroblast stages amongst the CD71high population was shifted implying that the erythroid lineage cells were overall more immature in the c-myb–/loxP fetal liver. Hence, there were relatively more Ter119–/low compared with Ter119high cells in c-myb–/loxP fetal liver than in the wild type (Figure 4B). Comparison of wild-type and c-mybloxP/loxP E15 fetal livers also showed a skewing of the distribution of early and late erythroblastic cells, although this was not as extreme as in the case of c-myb–/loxP (Figure 4B), and mature enucleated erythrocytes could be detected in touch preparations (Figure 3A). Reflecting the fact that the deficiency in haemopoiesis in c-mybloxP/loxP fetal liver seems to be compensated for to a large extent between E14 and E15 (compare with Figure 2B), a greater degree of imbalance in the erythroid lineage in c-mybloxP/loxP fetal liver was seen at E14 (Figure 4B).
Fig. 4. Erythroid differentiation is perturbed in the presence of reduced levels of c-Myb. (A and B) Flow cytometric analysis of fetal liver cells from wild-type, c-mybloxP/loxP, c-myb–/loxP and c-myb–/– embryos stained with Ter119–biotin and anti-CD71 followed by streptavidin–PE and anti-IgG1–FITC. The profiles in (A) are derived from E15 embryos. The summary histogram in (B) includes data from E15 embryos and also similar stainings of fetal livers from wild-type and c-mybloxP/loxP E14 embryos. (C and D) Culture of wild-type and c-mybloxP/loxP E14 fetal liver cells under conditions favouring expansion of erythroid precursors, maintaining a cell concentration of 1–3 × 106/ml. (C) The cumulative cell number. (D) Cultured cells were stained with Ter119-biotin and anti-CD71 as described in (A) at day 8.
The alterations in the relative numbers of cells at different stages of erythroid differentiation in embryos with one or two copies of the knockdown allele could be a result of perturbed entry into or progression through differentiation or of selective loss of cells by apoptosis. The situation in vivo is likely to be complicated by the system trying to compensate for deficiencies, as appears to happen in c-mybloxP/loxP embryos between E14 and E15. In order to get a clearer picture of how erythroid cell differentiation might be perturbed we cultured wild-type and c-mybloxP/loxP E14 fetal liver cells under selective serum-free conditions. The ability of the wild-type and c-mybloxP/loxP erythroid cells to expand was equivalent (Figure 4C). Interestingly, analysis of the expression of CD71 and Ter119 on cells within each population after 8 days of culture revealed a dramatic difference in the extent of differentiation (Figure 4D). Hence, the majority of wild-type cells were CD71high and Ter119low/high, representing the proerythroblast to basophilic erythroblast stage, whereas the c-mybloxP/loxP erythroid population was predominantly CD71highTer119–, characteristic of more immature cells roughly corresponding to CFU-E. Nevertheless, cells with a CD71highTer119low/high phenotype were present in the c-mybloxP/loxP culture indicating that further differentiation was possible, as might be expected from the presence of mature erythroid cells in embryos and adults. We found no evidence indicating any change in apoptosis in c-mybloxP/loxP erythroid cells expressing reduced levels of c-Myb either from cells taken directly from the fetal liver or in the erythroid cultures (data not shown).
Collectively, this analysis indicates that in the presence of low levels of c-Myb erythroid precursors are produced but their subsequent ability to differentiate is severely inhibited. A key point in the differentiation pathway affected by the level of c-Myb seems to be the transition from the CFU-E to the proerythroblast stage.
Lymphoid differentiation is particularly sensitive to the level of c-Myb
Since it has been shown previously that commitment to thymopoiesis can occur in the absence of c-Myb but that subsequent development is blocked (Allen et al., 1999) we wondered whether different levels of the protein would also differentially influence distinct stages of lymphopoiesis. Cells were collected from the thymus of wild-type, c-myb–/–, c-myb–/loxP and c-mybloxP/loxP E15 embryos and were stained for expression of CD44 and CD25 as an indicator of their state of development. Very few thymic cells could be obtained from c-myb–/– embryos and these were Thy1+CD44low in agreement with the very early block to thymopoiesis described by Allen et al. (1999) (data not shown). Greater numbers of cells, although still reduced relative to wild type, were obtained from the thymus of c-myb–/loxP embryos. These cells seemed to be blocked in their differentiation at the DN1 (CD44+CD25–) to DN2 (CD44+CD25+) stages (Figure 5A and Table II). Cells from c-mybloxP/loxP embryos were also much less abundant than in the wild type, although they had progressed further through differentiation compared with those from c-myb–/loxP embryos, the majority were at the DN2 (CD44+CD25+) stage showing little evidence of differentiation to the DN3 (CD44–CD25+) or DN4 (CD44–CD25–) stages (Figure 5A and Table II). Unlike wild-type thymocytes, which show some differentiation to the CD4+CD8+ stage at E16, there was little sign of this progression in c-mybloxP/loxP embryos (data not shown).
Since c-mybloxP/loxP embryos are capable of reaching birth we next investigated the extent of both thymopoiesis and B-lymphopoiesis in 6 day-old neonates. Comparing wild-type and c-mybloxP/loxP litter mates we again saw evidence of hindered differentiation at the DN2/3 stage in animals containing two knockdown c-myb alleles (Figure 5B). Some thymocytes had clearly progressed beyond the DN4 stage because we could detect CD4+8+ and a few CD4+ or CD8+ cells, although their number was severely reduced relative to the wild type. Analysis of the bone marrow revealed that B-cell development was also perturbed in animals expressing low levels of c-Myb. Hence, few B220+ cells could be detected and these were largely CD43+ suggesting that they were not maturing fully (Figure 5C). Examination of thymocytes from 3 month-old c-mybloxP/loxP mice indicated that, as seen for erythropoiesis, a near normal number and balance of lymphoid cell types can be attained (data not shown). To confirm that the c-mybloxP allele was still functioning as a knockdown in these circumstances we sorted CD4+8+ thymocytes from wild-type and c-mybloxP/loxP animals and performed a western blot analysis of c-Myb protein levels. As can be seen in Figure 5D, c-mybloxP/loxP CD4+8+ thymocytes expressed ∼20% of the level of c-Myb to that seen in equivalent wild-type cells.
Similar to our concern that the phenotype of erythroid cells expressing reduced levels of c-Myb could be influenced by stressed conditions resulting from the reduced cellularity, we wanted to ensure that the aberrant thymopoiesis seen in knockdown embryos was likewise cell autonomous. We therefore performed thymic organ cultures using deoxyguanosine-treated wild-type thymic lobes and mutant thymic precursors derived from either the E14 thymus or E15 fetal liver. Culturing of E14 wild-type thymocytes with wild-type thymic lobes for 7 days (Figure 6A) resulted in the expected maturation of cells as indicated by their expression of CD8 (70.3% positive), which represent cells of a CD4+CD8+ phenotype. In contrast, only a modest increase in cell number was seen in lobes seeded with E14 c-mybloxP/loxP thymocytes and few if any CD8+ cells were apparent. Wild-type thymocytes expanded ∼50-fold during culturing (from 103 to 5.6 ± 2.9 × 104), whereas the knockdown cells only increased by 4-fold (from 103 to 4 ± 3 × 103). The majority of the c-mybloxP/loxP cells were CD44+CD25– after 7 days while only a few CD44+CD25+ cells were present. This suggested that the knockdown thymocytes were not able to mature as efficiently to the DN2 stage or beyond compared with the situation seen in vivo. In case the cell intrinsic properties of the E14 thymocytes had been influenced by their being derived from a relatively acellular and aberrant environment, we decided to adopt the approach of seeding wild-type thymic lobes with more immature lymphoid progenitors derived from the fetal liver of wild-type or c-mybloxP/loxP E15 embryos. Fragments of fetal liver and deoxyguanosine-treated thymic lobes were cultured together separated by a membrane through which progenitors could migrate. Again, wild-type cells successfully seeded the thymic lobes and after culturing for 17 days >40% of the recoverable cells were CD8+ representing immature CD4+8+ thymocytes (Figure 6B and data not shown). Staining for CD44 and CD25 indicated the presence of more immature thymocytes, populations corresponding to DN2 and DN3 being discernible. Equivalent transfilter cultures of c-mybloxP/loxP fetal liver yielded fewer cells (3.3 ± 1.8 × 104 compared with 5.5 ± 0.8 × 105 with wild-type fetal liver) but mature thymocytes were clearly present (4.2% CD8+). The CD44/CD25 staining pattern also revealed progression through thymocyte differentiation, in fact the distribution of cell phenotypes was similar to that obtained for E15 thymuses (compare Figures 6B and 5A) in that a large proportion appeared ‘blocked’ at the DN2 stage. A relatively greater number of CD44+CD25– cells however were seen in the transfilter cultures compared with the in vivo situation and we are currently investigating whether these represent a second blocked DN1-like population or are CD44+ non-thymocytic cells.
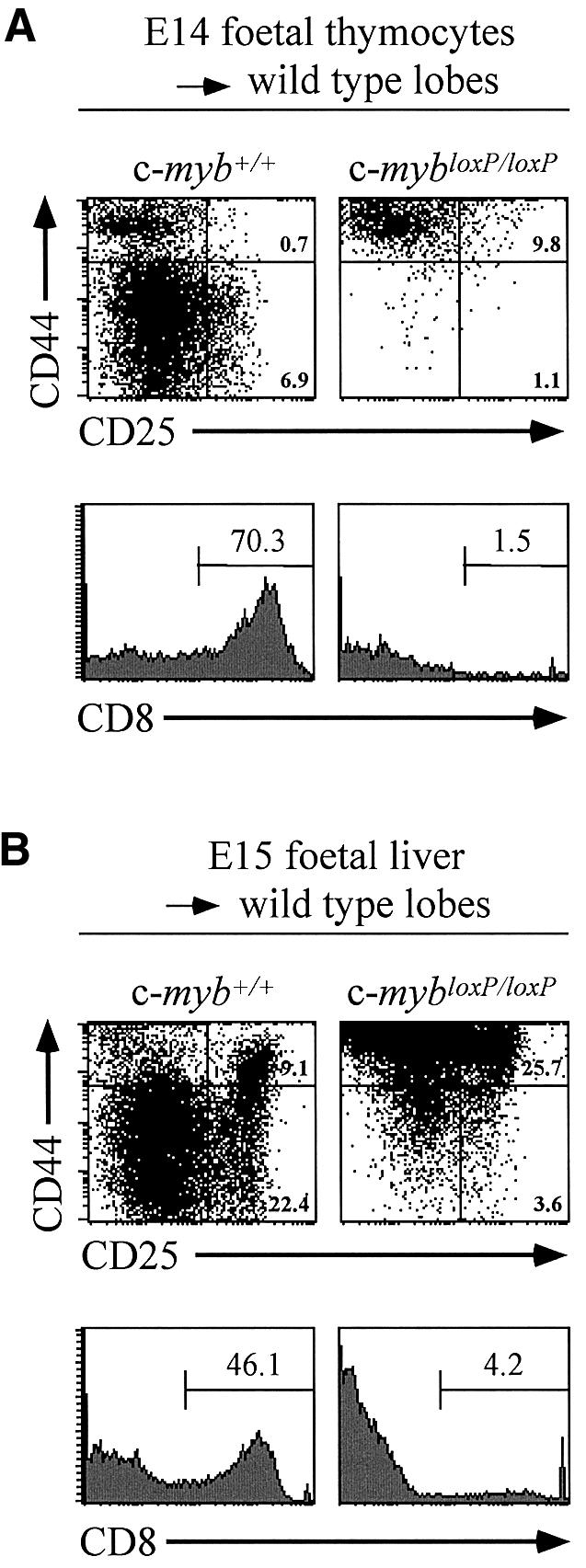
Fig. 6. Development of c-myb knockdown thymocytes in organ culture. Flow cytometric analysis of CD44 and CD25 expression (upper panels) and CD8 expression (lower panels) on cells derived from deoxyguanosine-treated thymic lobes seeded and subsequently cultured with thymocyte progenitors. (A) Cells from wild-type or c-mybloxP/loxP E14 thymuses were cultured with thymic lobes for 7 days. (B) Fragments of fetal liver from wild-type or c-mybloxP/loxP E15 embryos were incubated with thymic lobes separated by a 0.3 µm filter for 24 h. The fetal liver was then removed and the lobes cultured for an additional 16 days.
Discussion
The embryonic lethality resulting from homozygosity for the c-myb null allele dramatically illustrated the importance of c-Myb in the establishment of definitive haemopoiesis (Mucenski et al., 1991). Subsequent studies, using mice carrying this mutant allele, indicated that the absence of c-Myb did not prevent commitment to haemopoiesis, but rather prevented development of progenitor cells (Allen et al., 1999; Sumner et al., 2000). In order to further the investigation into the role of c-Myb in the development of specific lineages, or at later stages of haemopoiesis, we have created a loxP-modified c-myb allele (c-mybF), which can be used in combination with Cre recombinase to bring about controlled ablation. In a form retaining the neoR cassette (c-mybloxP) this new allele acts as a knockdown mutation, which leads to a reduction in c-Myb expression to ∼5–10% of the wild-type level.
By examining embryos carrying either the null allele and one copy of the knockdown allele, or two copies of the knockdown allele, it became apparent that progress through different stages of fetal liver haemopoiesis is dependent to different degrees on the level of c-Myb. The amount of c-Myb expressed from one knockdown allele is sufficient to allow expansion in vitro of committed progenitors, although their subsequent differentiation potential is altered. One manifestation of this altered differentiation potential leads to an absence of erythropoiesis, which in vivo results in embryonic lethality at much the same stage as in the case of c-myb–/– embryos. Homozygosity for the knockdown allele also results in perturbations in the differentiation potential of fetal liver progenitors although sufficient haemopoietic differentiation occurs that most embryos are born and reach adulthood.
Differential effects of reduced c-Myb levels on stage- and lineage-specific haemopoietic development
In embryos homozygous for the c-myb null allele, cells with a progenitor phenotype are produced in the AGM, and are initially present in the fetal liver, but behave aberrantly and are unable to expand normally (Sumner et al., 2000; J.Frampton and N.Emambokus, unpublished observations). The simplest explanation for these observations is that a major function of c-Myb is to control the proliferation of haemopoietic progenitor cells and that upon loss of c-myb there is a rapid decline in their numbers due to commitment to terminal differentiation in the absence of further expansion.
Low level c-Myb expression from the knockdown allele appears to be sufficient to allow progenitor expansion, although lineage commitment and differentiation is not correctly regulated. Hence, although equivalent numbers of functional progenitors are present in E13 fetal livers of wild-type and c-myb–/loxP or c-mybloxP/loxP embryos, a distinct profile of mature cell types is produced in in vitro colony assays. However, a decline in overall progenitor numbers in the c-myb–/loxP or c-mybloxP/loxP fetal livers between E13 and E15 suggests that uncontrolled differentiation might occur at the expense of progenitor expansion. Comparison of colonies at an early time point in the in vitro assays also indicated that multilineage differentiation of c-myb–/loxP or c-mybloxP/loxP progenitors occurs earlier than in wild-type cells. Additionally, changes in the cell content of the in vitro colonies indicate that the role played by c-Myb in the progression of differentiation is dependent on the lineage. Thus, progenitors from c-myb–/loxP or c-mybloxP/loxP fetal livers exhibit terminal differentiation along the megakaryocyte and monocyte lineages, whereas the erythroid lineage is apparently blocked at a specific point, implying that a particular threshold level of c-Myb is necessary in order for differentiation to proceed.
c-myb–/– embryos show signs of the beginnings of thymocyte development (CD44lo), which is in agreement with the observations of Allen et al. (1999) who looked at chimeras between c-myb–/– ES cells and rag1–/– blastocysts. The situation seen in the fetal thymus is akin to that seen for erythropoiesis, in that progression through early CD4–CD8– (DN) stages requires threshold levels of c-Myb; the level necessary for passage from DN2 to DN3 being higher than that for the DN1 to DN2 step. A specific block to thymopoiesis was also noted by Pearson and Weston (2000) in adult mice expressing a dominant negative c-Myb. In this case, cells were arrested at the DN3 stage subsequent to β-selection. Their failure to see effects earlier in thymopoiesis may be the result of the specificity or level of interference elicited by the transgenic Myb-engrailed protein or because of subtle differences between fetal and adult thymopoiesis.
Does c-Myb have an influence on commitment in myelopoiesis?
It was originally noted by Mucenski et al. (1991) that megakaryocytes are seen in the c-myb–/– fetal liver and it was proposed that normal megakaryopoiesis is possible in the absence of c-Myb. However, our subsequent study showed that megakaryopoiesis is aberrant in these circumstances and only mature cells are present (Sumner et al., 2000). This latter conclusion agrees with our observation of preferential differentiation along the megakaryocyte lineage in embryos with lower levels of c-Myb, both in terms of numbers of cells and in the behaviour of progenitor cells in CFU assays. Collectively, this suggests that a threshold level of c-Myb is required to prevent uncontrolled differentiation along the megakaryocyte lineage. Myelomonocytic differentiation appeared to be affected in a similar fashion in that unilineage monocytic colonies were elevated and macrophage were prevalent together with megakaryocytes in ‘mixed’ colonies. We noted a reduced number of neutrophil-containing colonies in assays of cells from c-myb–/loxP or c-mybloxP/loxP fetal livers although there was some evidence for the presence of neutrophils when cytospins of mixed colonies were examined.
It is interesting that a controlling effect of v-Myb on entry into thrombopoiesis and monocytic differentiation was previously noted in transformed avian multipotent progenitors and bipotent promyelocytes, respectively (Frampton et al., 1995). Upon induced inactivation of temperature-sensitive v-Myb (v-Mybts), transformed multipotent progenitors differentiated towards mature thrombocytes while bipotential promyelocytes differentiated exclusively towards macrophage. That granulocyte commitment could have been occurring in the latter case was suggested by the observation that co-expression of bcl-2 and v-Mybts resulted in the production of both granulocytes and monocytes when v-Myb was inactivated (Frampton et al., 1996).
The behaviour of the erythroid lineage in response to altered c-Myb levels appears different from cells committed to megakaryocytopoiesis and myelomonocytic differentiation. The absence of definitive red cells in c-myb–/– embryos implies either that c-Myb is required for commitment to the lineage, or for correct terminal differentiation. In contrast, embryos expressing reduced levels of c-Myb clearly undergo commitment to the erythroid lineage but exhibit alterations in the extent of differentiation depending on the amount of c-Myb. Analysis of the growth of c-mybloxP/loxP erythroid cells in ex vivo culture suggests that this lower level of c-Myb does not compromise proliferation but rather hinders the CFU-E to proerythroblast transition. However, terminal differentiation can occur and under the pressure of homeostatic mechanisms near normal numbers of cells are achieved in vivo. Earlier studies showed that constitutive expression of c-Myb in MEL cells (often equated with the CFU-E/proerythroblast) blocks inducible erythroid differentiation (Clarke et al., 1988). If the behaviour of this cell line can be compared to our in vivo and ex vivo observations, then this suggests that c-Myb downregulation is necessary at some point after the proerythroblast stage. c-myb–/loxP erythroid cells do not appear to be capable of terminal differentiation but can reach a stage corresponding to the basophilic erythroblast. This suggests that a specific level of c-Myb is necessary for passage beyond this point and that this is not reached when only one knockdown allele is expressed, but is just surpassed when the embryo is homozygous for the allele.
In conclusion, it would seem that the influence of c-Myb on myelopoiesis is mainly one of the regulation of cells at key stages, particularly at or near the point of lineage commitment, but also at some later stages of differentiation such as in erythroblasts. If c-Myb can be said to influence commitment decisions then this is most likely because the ‘barrier’ to differentiation along some lineages is lower, as might be the case for megakaryopoiesis.
Mechanisms underlying the differential influence of c-Myb on haemopoiesis
c-Myb has been implicated in the control of proliferation, cell survival and differentiation (for review see Oh and Reddy, 1999). Our work points to distinct requirements for c-Myb at different points in the haemopoietic hierarchy, these points seeming to correlate with those cells in which c-Myb is normally most highly expressed.
It is becoming recognized that the absolute level of a specific transcription factor is an important component of the mechanism of lineage specific regulation. One of the first examples of this came from experiments demonstrating that the level of ectopic expression of GATA-1 in chicken myelomonocytic cells determined the phenotype of the ‘reprogrammed’ cells (Kulessa et al., 1995). More recently, DeKoter and Singh (2000) showed that graded expression of PU.1 regulates B-cell and macrophage development, while Motohashi et al. (2000), were able to determine that the absolute level of the small maf proteins dictates the extent of maturation along the megakaryocyte lineage.
There are many ways in which variations in the level of a specific transcription factor might influence gene regulation differentially. Most likely, it is the consequence of distinct combinatorial interactions and the formation of multiprotein complexes. Such complexes may acquire the ability to regulate specific genes. c-Myb has a number of known partners with which it cooperates to regulate lineage-specific genes, for example C/EBPβ on myelomonocytic gene promoters and HES-1 on the CD4 gene (Ness et al., 1993; Allen et al., 2001). Alternatively, complex formation may effectively ‘titrate out’ particular factors and consequently have an indirect effect on gene regulation. In this way, the interaction between PU.1 and GATA-1 is a major determinant of erythroid versus myelomonocytic differentiation (Rektman et al., 1999). In a variant of this idea, c-Myb has been has been shown to be in ‘competition’ with GATA-1 for formation of a complex with CREB-binding protein (CBP) (Takahashi et al., 2000). This interaction between c-Myb and CBP may well underlie several of our observations with respect to the erythroid and megakaryocyte lineages. Hence, Kasper et al. (2002) recently showed that mutations in the protein interaction surface of the p300 co-activator have a profound effect on haemopoiesis, and that some of this effect may be mediated through an altered interaction with c-Myb.
c-Myb plays a unique role in the haemopoietic differentiation hierarchy. Though expressed in the haemopoietic stem cell and multi- and uni-lineage progenitors, the critical requirement for c-Myb appears to lie in the commitment of progenitor differentiation. It has often been thought that the role of c-Myb in lineage differentiation might be a default option upon cessation of cell proliferation. Although our results do indeed support this hypothesis, we also suggest that the role of c-Myb might also be instructive, with different threshold levels being required to affect the balance of cell fate.
Materials and methods
Generation and maintenance of floxed c-myb mice
A 129/SvEv mouse genomic library was screened to identify clones of the c-myb gene. LoxP sites upstream of exon 3 and downstream of exon 5, an FRT-flanked PGK-neo cassette and an HSV-tk cassette for negative selection were inserted as illustrated in Figure 1. The resulting targetting vector was linearized at a unique XhoI site. Further details of the cloning strategy can be obtained from the authors on request. Two floxed c-myb lines, 2C8 and 2J7, were generated and were indistinguishable. Mice were maintained on a C57/BL6 × 129Sv background.
Genotyping
Mice were genotyped by PCR analysis. The primers were: (i) For the intron 6 loxP site, ATCTGAAGAAAATGAATTGA and GCATCAGCTCGATGATAAGCA giving products of 233 bp and 281 bp from the wild-type and loxP-modified loci; and (ii) For the c-myb null allele, CCATGCGTCGCAAGGTGGAAC and TGGCCGCTTTTCTGGATTCATC giving an amplification product of 295 bp.
Colony assays and phenotypic cell analysis
Fetal liver cells (4 × 104) were plated in 2.5 ml 1% methylcellulose medium (Methocult M3434, Stem Cell Technologies, Vancouver) containing IMDM, 15% FCS, 0.1 mM 2-mercaptoethanol, 2 mM l-glutamine, 1% BSA, insulin (10 mg/ml), iron-saturated human transferrin (200 mg/ml), IL-3 (10 ng/ml), IL-6 (10 ng/ml), SCF (50 ng/ml), Epo (3 U/ml) and TPO (25 ng/ml). Cultures were incubated for 7 days at 37°C in a fully humidified 5% CO2 atmosphere and colony numbers were assessed between days 5 and 10. Single-cell preparations were cytospun onto slides and stained with May–Grünwald–Giemsa.
Erythroid cell culture
Expansion of predominantly erythroblasts in culture from E14 fetal liver cells was achieved by the method of Dolznig et al. (2001). A single cell suspension of fetal liver cells, treated to remove mature red cells, was grown at 1–3 × 106 cells/ml in serum-free StemPro-34 medium (Stem Cell Technologies) containing SCF (100 µg/ml), erythropoietin (2 U/ml) and dexamethasone (1 mM). Fresh media was added as required to maintain a roughly constant cell density.
Fetal thymic transfilter and thymocyte transfer assays
Thymic organ cultures were set up as described by Jenkinson et al. (1982). In brief, dissected lobes were placed on 0.8 µm pore size polycarbonate Nucleopore filters (Millipore), which were supported by squares of Artiwrap sponge (Medipost Ltd, Weymouth, UK). Culturing in the presence of 1.35 mM 2-deoxyguanosine (2-dGuo) for 5 days rendered the thymic lobes free of lymphoid cells. Prior to use in transfer assays, lobes were then washed extensively in RPMI 1640 containing 10% FCS to remove 2-dGuo. To establish transfilter assays, fragments of fetal liver were placed on the surface of 0.8 µm filters, onto which an additional filter of 3 µm pore size was placed. 2-dGuo treated lobes were then placed onto these 3 µm filters directly above the fetal liver fragments. Following 24 h culture, during which time haemopoietic precursors migrate from the liver fragments to the thymus lobes, 2-dGuo lobes were removed and placed onto 0.8 µm filters and maintained in DMEM containing 10% FCS. After the appropriate culture period, thymocytes were harvested from cultures by teasing apart the lobes with fine forceps, and viable cells counted. In experiments examining the development of mutant thymocyte precursors in wild-type 2-dGuo thymus lobes, thymocytes were placed directly onto the surface of individual lobes under standard organ culture conditions.
Antibodies, flow cytometry and cell sorting
Antibodies were used as FITC, PE or biotin conjugates or unconjugated in combination with an appropriate isotype-specific conjugated secondary antibody. With the exception of anti-rat IgG1-FITC (Serotec, UK), all unconjugated and conjugated antibodies were obtained from BD Pharmingen. Single cell suspensions of fetal liver or adult bone marrow, spleen and thymus were prepared by standard techniques. Red cells were depleted, when required, from the preparations by selective lysis and non-specific binding of antibodies to Fc receptors was prevented by use of anti-CD16/CD32 Fc-block (BD Pharmingen). Stained cells were analysed on a FACSCalibur flow cytometer using CellQuest software (Becton Dickinson).
CD4+8+ thymocytes were purified from 3 month-old thymuses after staining single cell suspensions (∼1.5 × 107 cells) with anti-CD4–PE and anti-CD8–FITC. Stained cells were sorted using a Cytomation MoFlo FACS machine.
Western blotting
E11 fetal livers were dissected and placed on ice. One hundred microlitres of lysis buffer (20 mM Tris, 7.4; 100 mM NaCl, 10 mM EDTA; 1 mM EGTA, 1% Triton X-100) containing 1 mM β-glycerophosphate, 5 mg/ml protease inhibitor cocktail and 1 mM vanadate was added, the livers resuspended with a 25-gauge needle and incubated on ice for 30 min. After centrifugation, the supernatant was collected and the protein concentration measured. Nuclear lysate (15 µg) was run on a 10% Laemmli gel and transferred onto Immobilon membrane. The membrane was blocked in 3% PBS + NFDM for 20 min, then incubated with 1 µg/ml anti-c-Myb clone 1.1 (Upstate) for 2.5 h at 21°C, washed twice in PBS and probed with a goat anti-mouse HRP (DAKO) at 1:2000 dilution for 1 h at 21°C. After washing the membrane twice in PBS and once in PBS + 0.05% Tween-20, the proteins were detected by enhanced chemiluminescence (ECL, Amersham).
CD4+8+ (4 × 106) thymocytes sorted from the thymuses of 3 month-old mice were extracted with lysis buffer, and 15 µg of protein run on a 10% Laemmli gel and transferred to membrane as described above. The membrane was probed with anti-c-Myb as described above and then after stripping with an HRP-conjugated anti β-actin monoclonal antibody (C3, Santa Cruz) followed by detection by ECL.
Acknowledgments
Acknowledgements
We would like to thank Holger Kulessa and Brigid Hogan for advice on ES targetting. We acknowledge Robert Sumner and Richard Coudroy for performing the blastocyst injections and Diane Fleary and Helen Partridge for animal husbandry. We are grateful to Stuart Orkin and Susan Dymecki for providing us with the GATA1-Cre and FLPe strains. N.E., A.V. and J.F. were supported by the Wellcome Trust. B.H., E.J. and G.A. were supported by the Medical Research Council.
References
- Allen R.D., Bender,T.P. and Siu,G. (1999) c-Myb is essential for early T cell development. Genes Dev., 13, 1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R.D., Kim,H.K., Sarafova,S.D. and Siu,G. (2001) Negative regulation of CD4 gene expression by a HES-1-c-Myb complex. Mol. Cell. Biol., 21, 3071–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M.F., Kukowska-Latallo,J.F., Westin,E., Smith,M. and Prochownik,E.W. (1988) Constitutive expression of a c-myb cDNA blocks friend murine erythroleukemia cell differentiation. Mol. Cell. Biol., 8, 884–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D., Vegiopoulos,A., Crawford,A., Mucenski,M., Bonifer,C. and Frampton,J. (2000) In vitro differentiation of c-myb–/– ES cells reveals that the colony forming capacity of unilineage macrophage precursors and myeloid progenitor commitment are c-Myb independent. Oncogene, 19, 3343–3351. [DOI] [PubMed] [Google Scholar]
- DeKoter R.P. and Singh,H. (2000) Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science, 288, 1439–1441. [DOI] [PubMed] [Google Scholar]
- Dolznig H., Boulme,F., Stangl,K., Deiner,E.M., Mikulits,W., Beug,H. and Mullner,E.W. (2001) Establishment of normal, terminally differentiated mouse erythroid progenitors: molecular characteriz ation of cDNA arrays. FASEB J., 15, 1442–1444. [DOI] [PubMed] [Google Scholar]
- Dymecki S.M. (1996). Flp recombinase promotes site-specific DNA recombination in embryonic stem cells and transgenic mice. Proc. Natl Acad. Sci. USA, 93, 6191–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ess K.C., Witte,D.P., Bascomb,C.P. and Aronow,B.J. (1999) Diverse developing mouse lineages exhibit high-level c-Myb expression in immature cells and loss of expression upon differentiation. Oncogene, 18, 1103–1111. [DOI] [PubMed] [Google Scholar]
- Frampton J., McNagny,K.M., Sieweke,M., Döderlein,G., Smith,G. and Graf,T. (1995) v-Myb DNA binding domain is required to block thrombocytic differentiation of Myb-Ets-transformed multipotent haematopoietic progenitors. EMBO J., 14, 2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton J., Ramqvist and Graf,T. (1996) v-Myb of E26 leukemia virus up-regulates bcl-2 and suppresses apoptosis in myeloid cells. Genes Dev., 10, 2720–2731. [DOI] [PubMed] [Google Scholar]
- Jasinski M., Keller,P., Fujiwara,Y., Orkin,S.H. and Bessler,M. (2001) GATA1-Cre mediates Piga gene inactivation in the erythroid/megakaryocytic lineage and leads to circulating red cells with a partial deficiency in glycosyl phosphatidylinositol-linked proteins (paroxysmal nocturnal hemoglobinuria type II cells). Blood, 98, 2248–2255. [DOI] [PubMed] [Google Scholar]
- Jenkinson E.J., Franchii,L.L., Kingston,R. and Owen,J.J.T. (1982) Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: applications for the production of chimaeric thymus rudiments. Eur. J. Immunol., 12, 583–587. [DOI] [PubMed] [Google Scholar]
- Kaspar L.H., Boussouar,F., Ney,P.A., Jackson,C.W., Rehg,J., van Deursen,J.M. and Brindle,P.K. (2002) A transcription-factor-binding surface of coactivator p300 is required for haematopoiesis. Nature, 419, 738–743. [DOI] [PubMed] [Google Scholar]
- Kastan M.B., Slamon,D.J. and Civin,C.I. (1989) Expression of protooncogene c-myb in normal human haemopoietic cells. Blood, 73, 1444–1451. [PubMed] [Google Scholar]
- Kulessa H., Frampton,J. and Graf,T. (1995) GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts and erythroblasts. Genes Dev., 9, 1250–1262. [DOI] [PubMed] [Google Scholar]
- Labastie M.C., Cortes,F., Romeo,P.H., Dulac,C. and Peault,B. (1998) Molecular identity of haemopoietic precursor cells emerging in the human embryo. Blood, 92, 3624–3635. [PubMed] [Google Scholar]
- Motohashi H., Katsuoka,F., Shavit,J.A., Engel,J.D. and Yamamoto,M. (2000) Positive or negative MARE-dependent transcriptional regulation is determined by the abundance of small Maf proteins. Cell, 103, 865–875. [DOI] [PubMed] [Google Scholar]
- Mucenski M.L., McLain,K., Kier,A.B., Swederlow,S.H., Schreiner,C.M., Miller,T.A., Pietryga,D.W., Scott,W.J.,Jr and Potter,S.S. (1991) A functional c-myb gene is required for normal murine foetal hepatic haemopoiesis. Cell, 65, 677–689. [DOI] [PubMed] [Google Scholar]
- Mukouyama Y.-S., Chiba,N., Mucenski,M.L., Satake,M., Miyajima,A., Hara,T. and Watanabe,T. (1999) Haemopoietic cells in cultures of the murine embryonic aorta–gonad–mesonephros region are induced by c-Myb. Curr. Biol., 9, 833–836. [DOI] [PubMed] [Google Scholar]
- Ness S.A., Kowenz-Leutz,E., Casini,T., Leutz,A. and Graf,T. (1993) Myb and NF-M: combinatorial activators of myeloid genes in heterologous cell types. Genes Dev., 7, 749–759. [DOI] [PubMed] [Google Scholar]
- Oh I.-H. and Reddy,E.P. (1999) The myb gene family in cell growth, differentiation and apoptosis. Oncogene, 18, 3017–3033. [DOI] [PubMed] [Google Scholar]
- Palis J., Robertson,S., Kennedy,M., Wall,C. and Keller,G. (1999) Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development, 126, 5073–5084. [DOI] [PubMed] [Google Scholar]
- Pearson R. and Weston,K. (2000) c-Myb regulates the proliferation of immature thymocytes following β-selection. EMBO J., 19, 6112–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rektman N., Radparvar,F., Evans,T. and Skoultchi,A.I. (1999) Direct interaction of haemopoietic transcription factors PU.1 and GATA-1: functional antagonism in erythroid cells. Genes Dev., 13, 1398–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitzmann J., Noben-Trauth,K. and Klempnauer,K.-H. (1995) Expression of mouse c-myb during embryonic development. Oncogene, 11, 2273–2279. [PubMed] [Google Scholar]
- Socolovsky M., Nam,H., Fleming,M.D., Haase,V.H., Brugnara,C. and Lodish,H.F. (2001) Ineffective erythropoiesis in Stat5a–/– 5b–/– mice due to decreased survival of early erythroblasts. Blood, 98, 3261–3273. [DOI] [PubMed] [Google Scholar]
- Sumner R., Crawford,A., Mucenski,M. and Frampton,J. (2000) Initiation of adult myelopoiesis can occur in the absence of c-Myb whereas subsequent development is strictly dependent on the transcription factor. Oncogene, 19, 3335–3342. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Suwabe,N., Dai,P., Yamamoto,M., Ishii,S. and Nakano,T. (2000) Inhibitory interaction of c-Myb and GATA-1 via transcriptional co-activator CBP. Oncogene, 19, 134–140. [DOI] [PubMed] [Google Scholar]